Thsi is a year old. But at 27 minutes David gets asked a couple fo “when” questions.
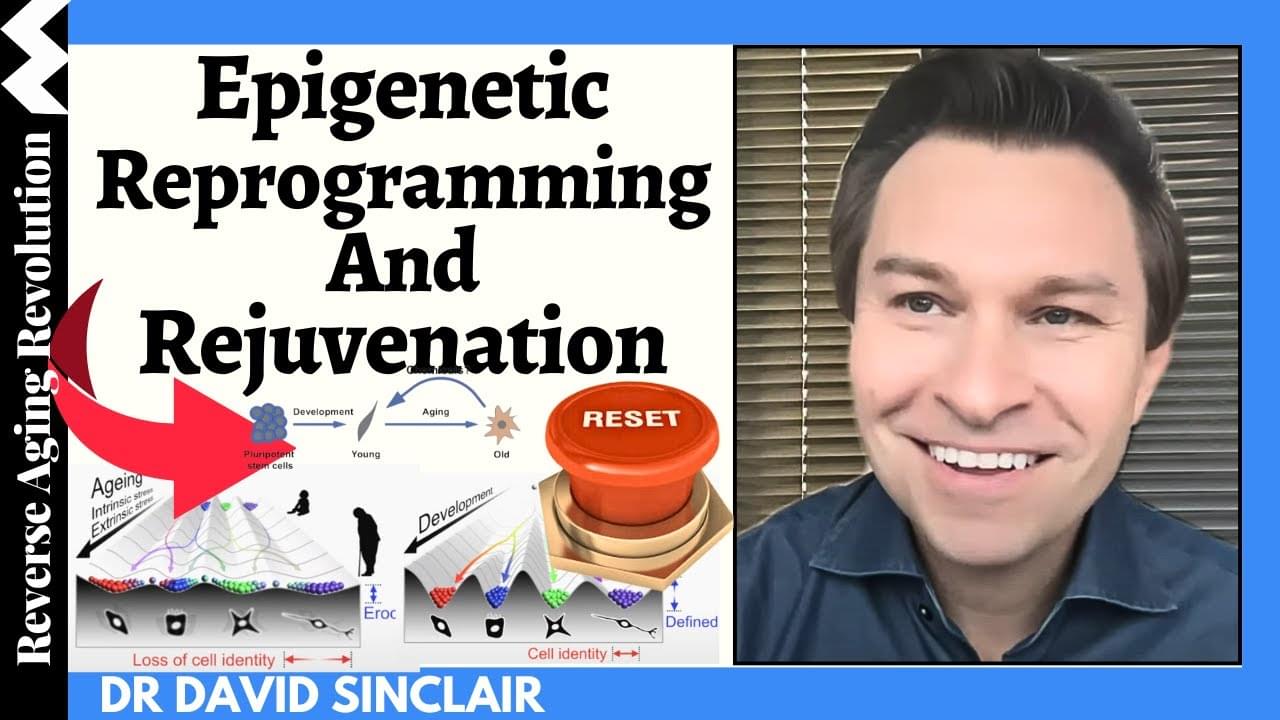
Dr. David Sinclair presents the progress of epigenetic reprogramming and rejuvenation in this video. He’s also answering questions on when he thinks the rejuvenation therapy be available in the Q\&A session at the end of the presentation.
00:54 Presentation.
25:42 Q\&A
David Sinclair is a professor in the Department of Genetics and co-director of the Paul F. Glenn Center for the Biology of Aging at Harvard Medical School, where he and his colleagues study sirtuins—protein-modifying enzymes that respond to changing NAD+ levels and to caloric restriction—as well as chromatin, energy metabolism, mitochondria, learning and memory, neurodegeneration, cancer, and cellular reprogramming.
Dr David Sinclair has suggested that aging is a disease—and that we may soon have the tools to put it into remission—and he has called for greater international attention to the social, economic and political and benefits of a world in which billions of people can live much longer and much healthier lives.