Women who have already passed through the menopause may be able to have children following a blood treatment usually used to heal wounds
Category: biotech/medical – Page 338
Gregg Braden Official
What if the most powerful organ in your body isn’t your brain, but your heart? In this deeply revealing compilation from Gaia’s MISSING LINK Series 👉 https://www.gaia.com/lp/mindful-maste…, Gregg Braden uncovers a forgotten truth buried in both science and ancient wisdom—that your heart holds 40,000 brain-like cells capable of memory, emotion, and thought.
Learn how you can unlock total recall, deep intuition, and spontaneous healing through harmonizing two forgotten systems: your heart and your brain.
00:00 – The Nightmare That Solved a Murder.
03:15 – Human Chromosome 2: Engineered Evolution?
07:30 – The Brain in the Heart: 40,000 Neurites.
11:00 – Transferred Memories in Organ Transplants.
16:20 – Little Girl’s Memory Solves a Crime.
21:15 – Heart Intelligence vs Brain Intelligence.
25:00 – Ancient Cultures & Heart-Based Education.
28:40 – Unlocking Superhuman Abilities.
32:20 – Total Recall & Intuition on Demand.
36:10 – Reprogramming the Subconscious.
39:00 – Heart-Brain Harmony Triggers 1,300 Biochemical Reactions.
Cherck Out Gregg’s latest book Pure Human: The Hidden Truth of Our Divinity, Power, and Destiny here 👉 https://hayhs.com/ph_pp_hc_az.
👍 LIKE if you enjoyed this video!
🔄 SHARE with your friends and spread the knowledge!
🔔 SUBSCRIBE for more amazing content!
👉 Hit the BELL Icon on our HOME Page to stay updated with our latest releases!
Stay Connected.
Official website: https://greggbraden.com/about-gregg-braden/
Facebook: https://www.facebook.com/GreggBraden.
Twitter: https://twitter.com/GreggBraden.
Instagram: https://www.instagram.com/Gregg.Braden/
This channel is managed by:
Zohar Entertainment Group International Inc, USA
YouTube Certified Enterprise Partner — FAM Networks, USA
Facebook: / greggbraden.
Twitter: / greggbraden.
Instagram: / gregg.braden


“It Seems Like Science Fiction”: Researchers Unleash Breakthrough Tracking Technology Using Environmental DNA
Scientists have revealed a novel means of tracking everything from wildlife to illicit substances using environmental DNA detectable in the air around us.
The findings, outlined in a new study published in Nature Ecology & Evolution, show that tracking virtually anything using environmental DNA can be achieved as simply as capturing this ever-present genetic material from the air using a vacuum.
The discovery, made by a team led by David Duffy, Ph.D., reveals DNA as a powerful new tool for detecting and tracking living organisms and a range of substances in virtually any environment.
CRISPR gene editing in blood stem cells linked to premature aging effects: Study offers solutions
Scientists at the San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), Milan, have found that gene editing using CRISPR-Cas9 in combination with AAV6 vectors can trigger inflammatory and senescence-like responses in blood stem cells, compromising their long-term ability to regenerate the blood system.
The study, published in Cell Reports Medicine, outlines new strategies to overcome this hurdle, improving both the safety and efficacy of gene-editing-based therapies for inherited blood disorders.
The research was led by Dr. Raffaella Di Micco, group leader at SR-Tiget, New York Stem Cell Foundation Robertson Investigator and Associate Professor at the School for Advanced Studies (IUSS) of Pavia, in collaboration with Professor Luigi Naldini, Director of SR-Tiget, and several European research partners.
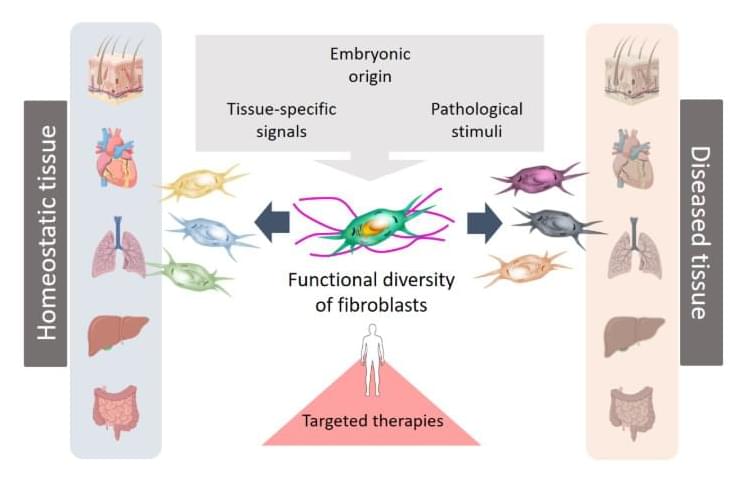
New perspectives for wound healing and the treatment of chronic diseases
Fibroblasts are specialised connective tissue cells that play a key role in wound healing and tissue regeneration. The recent scientific publication from the University of Leipzig Medical Center shows that fibroblasts respond differently depending on the organ and disease context. Their functions are shaped by their embryonic origin, tissue-specific signals, and pathological stimuli. These specialised cells are not only involved in tissue repair and remodelling, but also influence the immune system and the development of diseases such as cancer, fibrosis and chronic inflammatory conditions.
“Until now, our understanding of fibroblast diversity has been based primarily on studies in animal models. This new review is the first to compare and integrate extensive human studies that have used modern single-cell technologies. This approach makes it possible to combine findings from different human studies, creating a comprehensive picture of the various origins and functions of human fibroblasts,” says Professor Sandra Franz, lead author of the study from the University of Leipzig Medical Center.
This deeper understanding of cellular heterogeneity opens up new avenues for the development of targeted therapies.


The Revolution Against Aging And Death Festival (RAADFest): James Strole
New YT video, featuring RAADFest creator, James Strole!
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY