A study showed that chatbots alone outperformed doctors when making nuanced clinical decisions, but when supported by artificial intelligence, doctors performed as well as the chatbots.


A study showed that chatbots alone outperformed doctors when making nuanced clinical decisions, but when supported by artificial intelligence, doctors performed as well as the chatbots.
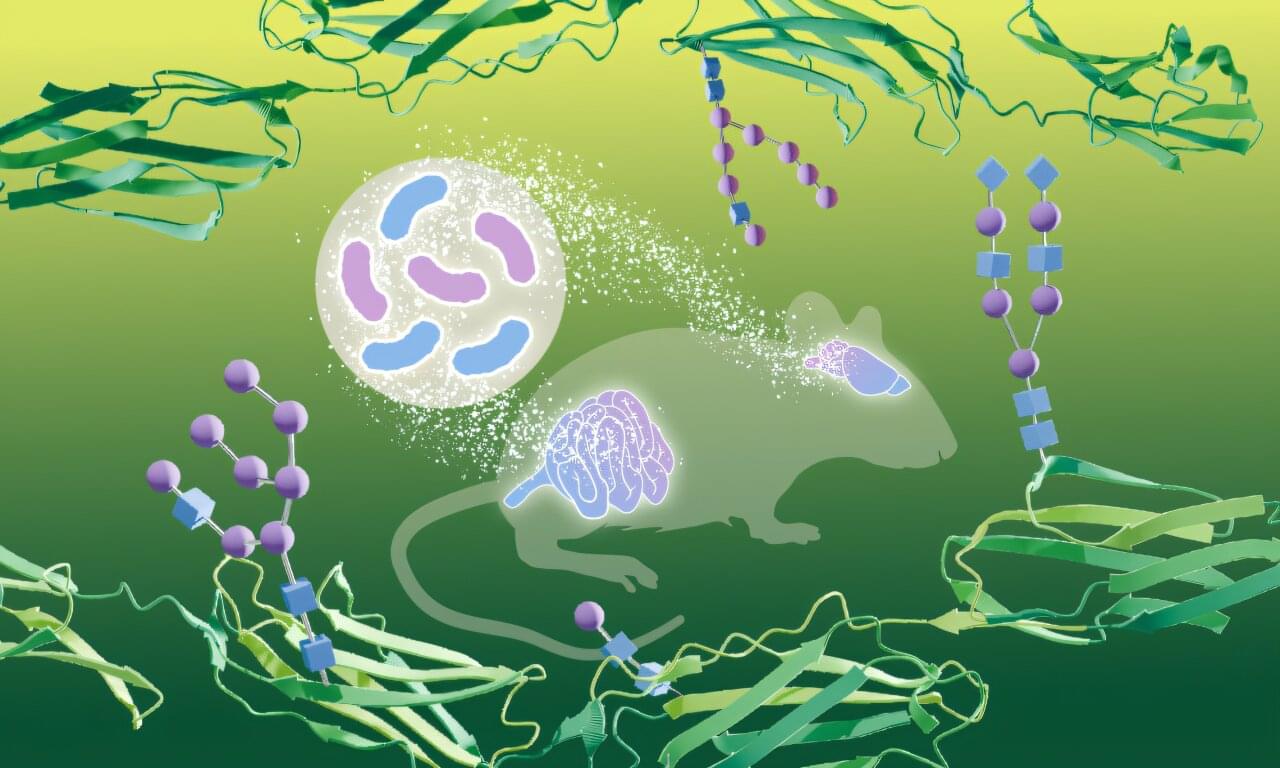
Our guts are home to trillions of bacteria, and research over the last few decades has established how essential they are to our physiology—in health and disease. A new study from EMBL Heidelberg researchers shows that gut bacteria can bring about profound molecular changes in one of our most critical organs—the brain.
The new study, published in the journal Nature Structural & Molecular Biology, is the first to show that bacteria living in the gut can influence how proteins in the brain are modified by carbohydrates—a process called glycosylation. The study was made possible by a new method the scientists developed—DQGlyco—which allows them to study glycosylation at a much higher scale and resolution than previous studies.
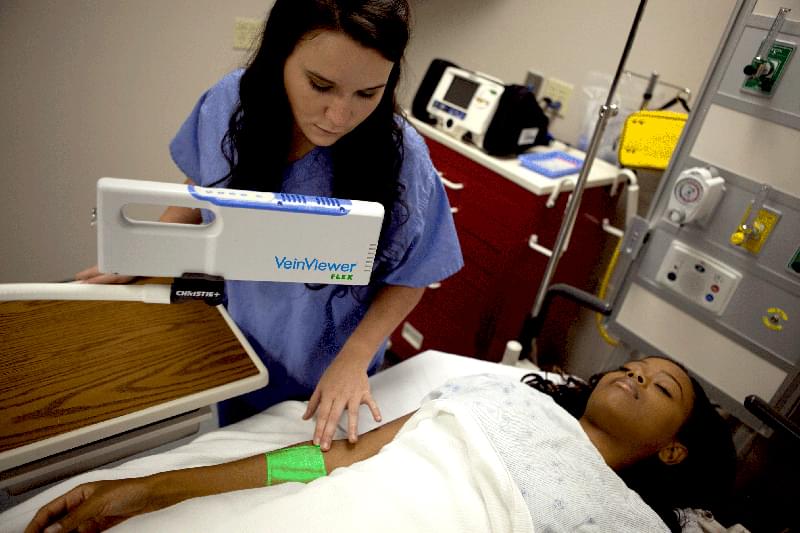
VeinViewer® Flex is a highly portable vascular access imaging device that can help you find the optimal venipuncture site and avoid potential complications.
With HD imaging and Df² technology, this small VeinViewer model is the brightest and only handheld vein illuminator that provides benefits for all patients during the entire Pre-, During-and Post-vascular access procedure. It is ideal for alternate care facilities, such as surgery and blood/plasma centers, as well as home healthcare and Emergency Medical Services (EMS), VeinViewer Flex is designed for durability and maximum portability. Flex is also suited for hospital departments such as the Emergency Department and NICU where space requirements and speed of assessment demand an ultra-portable and reliable vein finder.
How Does It Work?

A new mathematical model sheds light on how the brain processes different cues, such as sights and sounds, during decision making. The findings from Princeton neuroscientists may one day improve how brain circuits go awry in neurological disorders, such as Alzheimer’s, and could help artificial brains, like Alexa or self-driving car technology, more helpful.

Before joining MPFI, Wang was a research scientist at the Janelia Research Campus of Howard Hughes Medical Institute, working with Dr. Jeffery Magee and previously with Dr. Eva Pastalkova. At Janelia, she studied the hippocampal neuronal activities that represent memory traces. In particular, she employed memory tasks that can reversibly toggle the influence of sensory inputs on and off and isolated neuronal activities associated with internally stored memory.
Wang was trained as an electrical engineer. She completed her graduate study under the mentorship of Drs. Shih-Chii Liu, Tobi Delbruck and Rodney Douglas at the Swiss Federal Institute of Technology Zurich (ETHZ). During her Ph.D. training, she designed brain-inspired computational systems on silicon chips. These fully reconfigurable systems incorporated electronic circuits of a network of neurons with dendrites and synapses. Using these systems as simulation tools, she also investigated the computational principles native to a neuron with active dendrites.

Gardenias are known for their rich, earthy fragrance, waxy petals and brilliant white color that contrasts with the deep emerald green of their leaves. The plant has long been prized by herbalists, seekers of food and fabric dyes, and even pharmaceutical companies.
Now, a collaborative team of scientists at several research centers in the United States has found that a compound known as genipin, derived from the gardenia plant called Cape jasmine, can prompt nerve regeneration. Neurons damaged and stunted by disease find new life in the lab when exposed to the plant-derived compound.
The chemical comes from the fruit of this extraordinarily versatile plant. Gardenia shrubs, in general, are native to tropical and subtropical regions of Asia. But the plants are propagated globally by horticulturists and amateur gardeners who are most familiar with the flower’s beauty and the intoxicating scent of their perfume.

Years before tau tangles show up in brain scans of patients with Alzheimer’s disease, a biomarker test developed at the University of Pittsburgh School of Medicine can detect small amounts of the clumping-prone tau protein and its misfolded pathological forms that litter the brain, cerebrospinal fluid and potentially blood, new research published today in Nature Medicine suggests.
The cerebrospinal fluid biomarker test correlates with the severity of cognitive decline, independent of other factors, including brain amyloid deposition, thereby opening doors for early-stage disease diagnosis and intervention.
Since amyloid-beta pathology often precedes tau abnormalities in Alzheimer’s disease, most biomarker efforts have focused on early detection of amyloid-beta changes. However, the clumping of tau protein into well-ordered structures referred to by pathologists as “neurofibrillary tangles” is a more defining event for Alzheimer’s disease as it is more strongly associated with the cognitive changes seen in affected people.
As temperatures drop, norovirus cases increase and more of its RNA ends up in sewage. This year, wastewater samples in the United States show norovirus levels have already passed the previous two seasonal peaks.
The “Ferrari of viruses” is having a banner season. Norovirus, which races through cruise ships, homes, and long-term care facilities, is experiencing a remarkable winter surge in the Northern Hemisphere, sending large numbers of people racing to the bathroom and many others to the hospital, and in rare cases, proving fatal. In the United States, for example, 91 outbreaks of the intestinal virus occurred in the first week of December 2024, far above the previous maximum, 65, for the same week between 2010 and 2024. And levels of its genes in U.S. wastewater are an order of magnitude above last year.
“The early data for the early part of the season is certainly supporting that we’re going to have a pretty intense norovirus year,” says Lisa Lindesmith, who studies the virus at the University of North Carolina (UNC) at Chapel Hill. Some of the surge may be due to a new variant of the virus, unfamiliar to many people’s immune systems, and the resumption of cruises and other gatherings that the COVID-19 pandemic interrupted. And there’s no vaccine anywhere in sight: The most advanced candidate just failed a key trial and others won’t be ready for several years.
Norovirus thrives in cold climes, causing explosive diarrhea and vomiting that typically only last for a day. But several weeks after people recover, they can still shed the virus, and it can remain infectious for long periods on surfaces. It’s notoriously resistant to many disinfectants, and studies in adult volunteers have shown just a trace of virus is enough to sicken a person. Oysters are also a source of infection, because the filter-feeding mollusks concentrate the virus from contaminated water in their tissues. U.S. health officials issued several warnings about infected oysters in December, and France has banned oyster harvesting in certain regions because of norovirus outbreaks.
The interaction between cellular senescence and cancer is complex and multifaceted, senescence can both promote and inhibit tumor progression through various mechanisms. M6A methylation modification regulates the aging process of cells and tissues by modulating senescence-related genes. In this review, we comprehensively discuss the characteristics of cellular senescence, the signaling pathways regulating senescence, the biomarkers of senescence, and the mechanisms of anti-senescence drugs. Notably, this review also delves into the complex interactions between senescence and cancer, emphasizing the dual role of the senescent microenvironment in tumor initiation, progression, and treatment. Finally, we thoroughly explore the function and mechanism of m6A methylation modification in cellular senescence, revealing its critical role in regulating gene expression and maintaining cellular homeostasis. In conclusion, this review provides a comprehensive perspective on the molecular mechanisms and biological significance of cellular senescence and offers new insights for the development of anti-senescence strategies.
Cellular senescence is a complex and multifaceted biological process characterized by a stable arrest of the cell cycle in response to various stressors, such as DNA damage, oxidative stress, and oncogene activation (1). Although senescent cells no longer proliferate, they remain metabolically active and exhibit distinct phenotypic changes, including the secretion of pro-inflammatory factors, collectively termed the senescence-associated secretory phenotype (SASP) (2, 3). Senescence plays dual roles in physiological and pathological contexts: it is essential for processes like tissue remodeling, wound healing, and tumor suppression, yet its accumulation contributes to aging, chronic inflammation, and the progression of age-related diseases, including cancer and neurodegenerative disorders (4). Understanding the mechanisms underlying cellular senescence is crucial for developing therapeutic strategies to harness its beneficial aspects while mitigating its detrimental effects.
Scientists have provided a diagnosis for more than 500 European patients who did not know their condition. This work, which was performed by the Solving the Unsolved Rare Diseases (Solve-RD) consortium and was highly collaborative, has been reported in Nature Medicine.
In the European Union, a rare disorder is defined as one that occurs in fewer than five of 10,000 people. Genetic mutations are the cause of most of these rare disorders, but genetic sequencing cannot always provide an easy answer.