Regulator says certain types of pet food are more frequently connected to heart disease in dogs, but why is unclear.



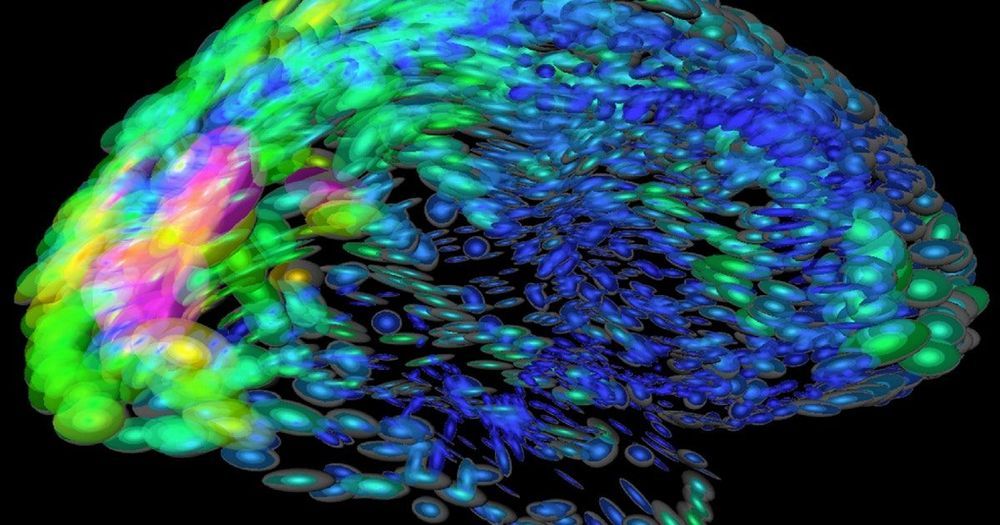
A new mouse study highlights the proteins responsible for LC3-associated endocytosis (LANDO), an autophagy process that is involved in degrading β-amyloid, the principal substance associated with Alzheimer’s disease.
Proteostasis
Proteins in the human brain can form misfolded, non-functional, and toxic clumps known as aggregates. Preventing these aggregates from forming, and removing them when they do, is a natural function of the human body, and it is known as proteostasis. However, as we age, this function degrades, and loss of proteostasis is one of the hallmarks of aging. The resulting accumulation of aggregates leads to several deadly diseases, one of which is Alzheimer’s.


HUNTERSVILLE, N.C. (WCNC) – A North Carolina couple couldn’t bear to break the bond they had with their furry feline friend. So after 19-year-old Cinnabun passed away, the Bullerdicks decided to clone their kitty.
The cost? A whopping price of $25,000.
The couple found a Texas-based company known for cloning dogs, cats and horses. They bought a kit and with a skin sample and saliva sample… Cinnabun the second was born.

Researchers from Lund University, together with the Roche pharmaceutical company, have developed a method to create a new blood marker capable of detecting whether or not a person has Alzheimer’s disease. If the method is approved for clinical use, the researchers hope eventually to see it used as a diagnostic tool in primary healthcare. This autumn, they will start a trial in primary healthcare to test the technique.
Currently, a major support in the diagnostics of Alzheimer’s disease is the identification of abnormal accumulation of the substance beta-amyloid, which can be detected either in a spinal fluid sample or through brain imaging using a PET scanner.
“These are expensive methods that are only available in specialist healthcare. In research, we have therefore long been searching for simpler diagnostic tools,” says Sebastian Palmqvist, associate professor at the unit for clinical memory research at Lund University, physician at Skåne University Hospital and lead author of the study.

Could be used in a portable device to genetically reprogram ones body.
Environmental conditions, such as heat, acidity, and mechanical forces, can affect the behavior of cells. Some biologists have even shown that magnetic fields can influence them. Now, for the first time, an international team reports that low-strength magnetic fields may foster the reprogramming of cellular development, aiding in the transformation of adult cells into pluripotent stem cells (ACS Nano 2014, DOI: 10.1021/nn502923s). If confirmed, the phenomenon could lead to new tools for bioengineers to control cell fates and help researchers understand the potential health effects of changing magnetic fields on astronauts.
Biologists have been building up evidence that magnetic fields affect living things, says Michael Levin, director of Tufts University’s Center for Regenerative & Developmental Biology, who was not involved in the new study. For example, plants and amphibian embryos develop abnormally when shielded from Earth’s geomagnetic field. And there’s some clinical evidence that particular electromagnetic frequencies promote bone fracture healing and wound repair (Eur. Cytokine Network 2013, DOI: 10.1684/ecn.2013.0332).
“It’s been a huge unknown how a cell senses electromagnetic fields and then translates that into a change in identity or a change in gene expression,” says Christopher J. Lengner, a cell biologist at the University of Pennsylvania. He worked with a group of bioengineers led by Jongpil Kim of Dongguk University, in Seoul, South Korea, to see if these fields could influence a process they were all interested in: reprogramming a cell’s developmental state.

With the end of the Vietnam and Cold wars, Jason members began to branch out from physics and engineering. In 1977, they did their first assessment of global climate models and later advised DOE on which atmospheric measurements were most critical for the models. Since the mid-1990s, Jason has studied biotechnologies, including techniques for detecting biological weapons.
After near-death experience, top scientists seek a long-term home in the U.S. government.

Soon, you might not need anything more specialized than a readily accessible touchscreen device and any existing data sets you have access to in order to build powerful prediction tools. A new experiment from MIT and Brown University researchers have added a capability to their ‘Northstar’ interactive data system that can “instantly generate machine-learning models” to use with their exiting data sets in order to generate useful predictions.
One example the researchers provide is that doctors could make use of the system to make predictions about the likelihood their patients have of contracting specific diseases based on their medial history. Or, they suggest, a business owner could use their historical sales data to develop more accurate forecasts, quickly and without a ton of manual analytics work.
Researchers are calling this feature the Northstar system’s “virtual data scientist,” (or VDS) and it sounds like it could actually replace the human equivalent, especially in settings where one would never actually be readily available or resourced anyway. Your average doctor’s office doesn’t have a dedicated data scientist headcount, for instance, and nor do most small- to medium-sized businesses for that matter. Independently owned and operated coffee shops and retailers definitely wouldn’t otherwise have access to this kind of insight.