Reluctant to pop a paracetamol for that headache? Virtual reality might offer an alternative solution.


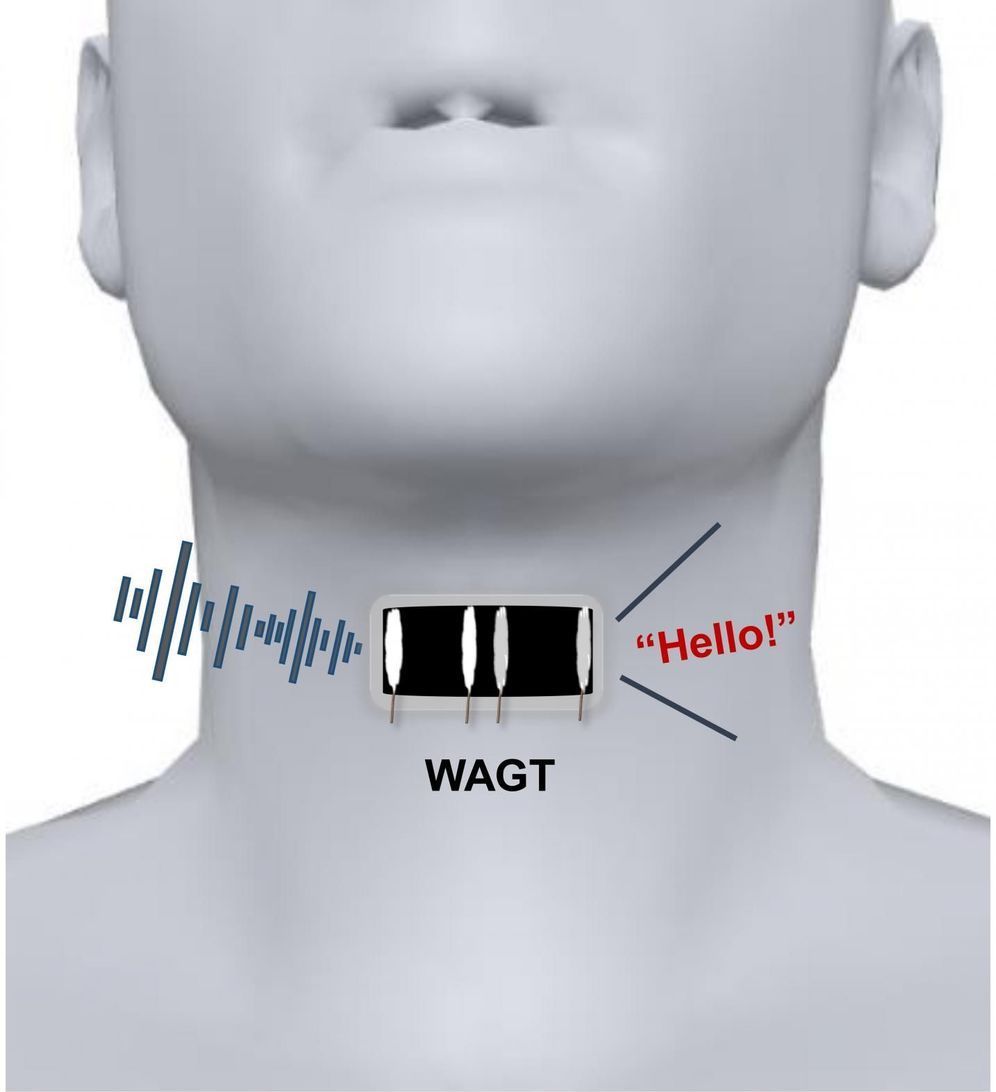
Most people take speech for granted, but it’s actually a complex process that involves both motions of the mouth and vibrations of folded tissues, called vocal cords, within the throat. If the vocal cords sustain injuries or other lesions, a person can lose the ability to speak. Now, researchers reporting in ACS Nano have developed a wearable artificial throat that, when attached to the neck like a temporary tattoo, can transform throat movements into sounds.
Scientists have developed detectors that measure movements on human skin, such as pulse or heartbeat. However, the devices typically can’t convert these motions into sounds. Recently, He Tian, Yi Yang, Tian-Ling Ren and colleagues developed a prototype artificial throat with both capabilities, but because the device needed to be taped to the skin, it wasn’t comfortable enough to wear for long periods of time. So the researchers wanted to develop a thinner, skin-like artificial throat that would adhere to the neck like a temporary tattoo.
To make their artificial throat, the researchers laser-scribed graphene on a thin sheet of polyvinyl alcohol film. The flexible device measured 0.6 by 1.2 inches, or about double the size of a person’s thumbnail. The researchers used water to attach the film to the skin over a volunteer’s throat and connected it with electrodes to a small armband that contained a circuit board, microcomputer, power amplifier and decoder. When the volunteer noiselessly imitated the throat motions of speech, the instrument converted these movements into emitted sounds, such as the words “OK” and “No.” The researchers say that, in the future, mute people could be trained to generate signals with their throats that the device would translate into speech.
Technology that translates cortical activity into speech would be transformative for people unable to communicate as a result of neurological impairment. Decoding speech from neural activity is challenging because speaking requires extremely precise and dynamic control of multiple vocal tract articulators on the order of milliseconds. Here, we designed a neural decoder that explicitly leverages the continuous kinematic and sound representations encoded in cortical activity to generate fluent and intelligible speech. A recurrent neural network first decoded direct cortical recordings into vocal tract movement representations, and then transformed those representations to acoustic speech output. Modeling the articulatory dynamics of speech significantly enhanced performance with limited data. Naïve listeners were able to accurately identify and transcribe decoded sentences. Additionally, speech decoding was not only effective for audibly produced speech, but also when participants silently mimed speech. These results advance the development of speech neuroprosthetic technology to restore spoken communication in patients with disabling neurological disorders.
TARRYTOWN, N.Y., Aug. 12, 2019 /PRNewswire/ — Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) today announced that the Company was informed by study investigators that a randomized, controlled trial evaluating four investigational therapies for Ebola virus infection was stopped early because.

US scientists have begun the daunting task of trying to work out how many genes there are in the human microbiome.
Even when you consider just the gut and the mouth (in itself, a unique research double) the numbers are potentially overwhelming.
Microbiologists and bioinformaticians from Harvard Medical School and Joslin Diabetes Centre gathered all publicly available sequencing data on human oral and gut microbiomes and analyzed the DNA from around 3500 samples – 1400 from mouths and 2100 from guts.
Last month, Elon Musk’s Neuralink, a neurotechnology company, revealed its plans to develop brain-reading technology over the next few years. One of the goals for Musk’s firm is to eventually implant microchip-devices into the brains of paralyzed people, allowing them to control smartphones and computers.
Although this Black Mirror-esque technology could hold potentially life-changing powers for those living with disabilities, according to Cognitive Psychologist Susan Schneider, it’s not such a great idea, and I can’t help but feel relieved, I’m with Schneider on this.

The Food and Drug Administration on Wednesday approved a new antibiotic that, when combined with two existing antibiotics, can tackle the most formidable and deadly forms of tuberculosis. The trio of drugs treats extensively drug-resistant tuberculosis (XDR-TB), along with cases of multidrug-resistant tuberculosis (MDR-TB) that have proven unresponsive to other treatments.
Tuberculosis is the single leading infectious killer in the world, infecting an estimated 10 million people in 2017 and killing 1.6 million of them. XDR-TB and MDR-TB are even more savage forms of the disease, which is caused by the bacterium Mycobacterium tuberculosis. The drug-resistant strains of TB kill an estimated 60% and 40% of their victims, respectively.
MDR-TB strains can resist at least the two most powerful anti-TB drugs, isoniazid and rifampin. A strain gets into XDR-territory when they also become resistant to any fluoroquinolone drug, such as ciprofloxacin or levofloxacin, plus at least one of three injectable second-line drugs, which are amikacin, kanamycin, and capreomycin. Drug-resistant strains of tuberculosis infected an estimated 558,000 people in 2017.


A team of scientists at UC San Francisco and the National Institutes of Health have achieved another CRISPR first, one which may fundamentally alter the way scientists study brain diseases.
In a paper published August 15 in the journal Neuron, the researchers describe a technique that uses a special version of CRISPR developed at UCSF to systematically alter the activity of genes in human neurons generated from stem cells, the first successful merger of stem cell-derived cell types and CRISPR screening technologies.
Though mutations and other genetic variants are known to be associated with an increased risk for many neurological diseases, technological bottlenecks have thwarted the efforts of scientists working to understand exactly how these genes cause disease.