A gene that appeared sometime after humans began processing their food seems to protect some people from type 2 diabetes.



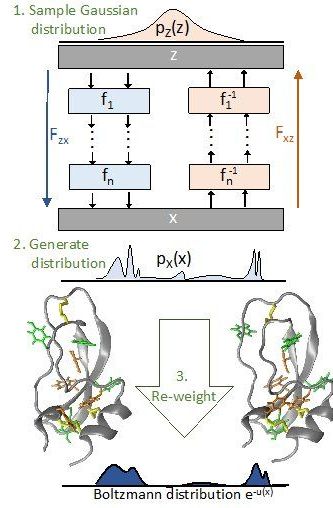
A team of scientists at Freie Universität Berlin has developed an Artificial Intelligence (AI) method that provides a fundamentally new solution of the “sampling problem” in statistical physics. The sampling problem is that important properties of materials and molecules can practically not be computed by directly simulating the motion of atoms in the computer because the required computational capacities are too vast even for supercomputers. The team developed a deep learning method that speeds up these calculations massively, making them feasible for previously intractable applications. “AI is changing all areas of our life, including the way we do science,” explains Dr. Frank Noé, professor at Freie Universität Berlin and main author of the study. Several years ago, so-called deep learning methods bested human experts in pattern recognition—be it the reading of handwritten texts or the recognition of cancer cells from medical images. “Since these breakthroughs, AI research has skyrocketed. Every day, we see new developments in application areas where traditional methods have left us stuck for years. We believe our approach could be such an advance for the field of statistical physics.” The results were published in Science.
Statistical Physics aims at the calculation of properties of materials or molecules based on the interactions of their constituent components—be it a metal’s melting temperature, or whether an antibiotic can bind to the molecules of a bacterium and thereby disable it. With statistical methods, such properties can be calculated in the computer, and the properties of the material or the efficiency of a specific medication can be improved. One of the main problems when doing this calculation is the vast computational cost, explains Simon Olsson, a coauthor of the study: “In principle we would have to consider every single structure, that means every way to position all the atoms in space, compute its probability, and then take their average. But this is impossible because the number of possible structures is astronomically large even for small molecules.

Researchers have designed a machine learning algorithm that predicts the outcome of chemical reactions with much higher accuracy than trained chemists and suggests ways to make complex molecules, removing a significant hurdle in drug discovery.
University of Cambridge researchers have shown that an algorithm can predict the outcomes of complex chemical reactions with over 90% accuracy, outperforming trained chemists. The algorithm also shows chemists how to make target compounds, providing the chemical “map” to the desired destination. The results are reported in two studies in the journals ACS Central Science and Chemical Communications.
A central challenge in drug discovery and materials science is finding ways to make complicated organic molecules by chemically joining together simpler building blocks. The problem is that those building blocks often react in unexpected ways.
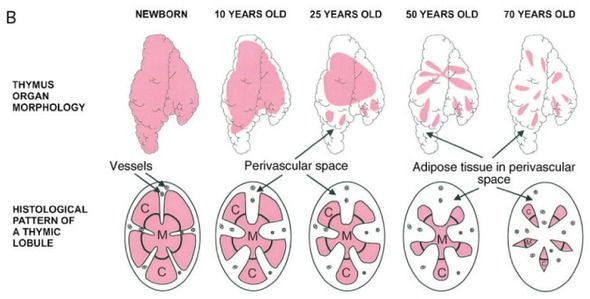
Yesterday, the TRIIM study was described in science news headlines around the world, though, through a glitch, the original research paper is not yet on the Aging Cell web site. (You saw it first here.) I refer you to the writeup in Nature’s News section for a full summary of the paper, and in this column I will add my personal framing, and what I know about the study from private connection to its authors and one of the subjects. The big news is setback of the epigenetic clock, by several methylation measures. Instead of getting a year older during the trial, nine subjects got a year younger, on average, based on the version of the Horvath methylation clock that best predicts lifespan. The study had been originally designed to regrow the thymus. (Loss of thymus function has been linked to the collapse of the immune system that occurs typically before age 70.) Imaging showed that the functional part of the thymus expanded over the course of the trial, and blood tests confirmed improved immune function. The treatment included.
Go modular or even get an upgrade:
A team of researchers from Carnegie Mellon University just 3D printed functional components of the human heart — including small blood vessels and large beating ventricles.
“We now have the ability to build constructs that recapitulate key structural, mechanical, and biological properties of native tissues,” said Adam Feinberg, a professor at Carnegie Mellon and the co-founder of 3D printing company FluidForm, which built the tech the team used, in a statement.
Lub Dub
Printing tissues capable of functioning like the real thing is particularly challenging. Complex shapes have to be supported as they’re being printed or otherwise they begin to sag. The team solved this issue, according to a paper published in the journal Science today, by printing scaffolds from a temporary support gel.


WASHINGTON, D.C.-Today, U.S. Secretary of Energy Rick Perry announced the establishment of the DOE Artificial Intelligence and Technology Office (AITO). The Secretary has established the office to serve as the coordinating hub for the work being done across the DOE enterprise in Artificial Intelligence. This action has been taken as part of the President’s call for a national AI strategy to ensure AI technologies are developed to positively impact the lives of Americans.
DOE-fueled AI is already being used to strengthen our national security and cybersecurity, improve grid resilience, increase environmental sustainability, enable smarter cities, improve water resource management, as well as speed the discovery of new materials and compounds, and further the understanding, prediction, and treatment of disease. DOE’s National Labs are home to four of the top ten fastest supercomputers in the world, and we’re currently building three next-generation, exascale machines, which will be even faster and more AI-capable computers.
“The world is in the midst of the Golden Age of AI, and DOE’s world class scientific and computing capabilities will be critical to securing America’s dominance in this field,” said Secretary Perry. “This new office housed within the Department of Energy will concentrate our existing efforts while also facilitating partnerships and access to federal data, models and high performance computing resources for America’s AI researchers. Its mission will be to elevate, accelerate and expand DOE’s transformative work to accelerate America’s progress in AI for years to come.”

NEW YORK (Reuters Health) — A convolutional neural network trained through deep learning can accurately predict a person’s age and gender using only standard 12-lead ECG signals, researchers report.
“Our standard diagnostic tools may have far more information behind them than we’ve come to expect throughout standard approaches to diagnostic interpretation,” said Dr. Suraj Kapa from Mayo Clinic College of Medicine, in Rochester, Minnesota.
“Between this study and other prior studies showing that we can predict likelihood of having atrial fibrillation from a normal sinus ECG or the presence of a low ejection fraction, AI-enabled ECG analysis may offer new, rapid, and cost-effective insights into human health well beyond what we could have anticipated in the last two centuries since the ECG was first developed,” he told Reuters Health by email.