Most advanced-stage cancers mutate, resisting drugs meant to kill them. Now doctors are harnessing the principles of evolution to thwart that lethal adaptation.



Today’s efforts in AI are somewhat less flashy, though still potentially revolutionary, and all seem to recognize one vital lesson: treating patients is both art and science. Rather than attempting to replace the physicians in medical practice, AI can, and should, say more experts, become a valuable tool in enhancing what doctors do.
Here’s where AI in medicine excels — and where it doesn’t yet measure up.

During the Great Embryonic Stem Cell Debate, circa 2001–2008, I watched “the scientists” blatantly lie about the supposedly low potential for adult stem cells and the CURES! CURES! CURES that were just around the corner from embryonic stem cells. You remember: Children would soon be out of their wheelchairs and Uncle Ernie’s Parkinson’s would soon be a disease of the past.
The pro-ESCR campaign was filled with so much disinformation and hype — willingly swallowed by an in-the-tank media — all in a corrupt attempt to overturn the minor federal funding restrictions over ESCR imposed by the president, and to hurt President Bush politically.
What kind of blood do we want in our bodies?
#wthfilm #plantbased #wfpb #health #cancer #goplantbased


Revolutions is a series that brings together a hand-picked selection of recent articles canvassing cutting-edge insights into major scientific advances. This installment brings you up to date with the ground-breaking new discoveries made around the regenerative possibilities of induced pluripotent stem cells, which can theoretically be coaxed into any kind of cell in the human body.
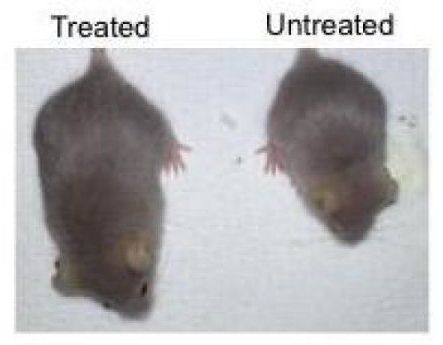
The findings, published on February 18, 2019 in the journal Nature Medicine, highlight a novel CRISPR/Cas9 genome-editing therapy that can suppress the accelerated aging observed in mice with Hutchinson-Gilford progeria syndrome, a rare genetic disorder that also afflicts humans. This treatment provides important insight into the molecular pathways involved in accelerated aging, as well as how to reduce toxic proteins via gene therapy.
“Aging is a complex process in which cells start to lose their functionality, so it is critical for us to find effective ways to study the molecular drivers of aging,” says Juan Carlos Izpisua Belmonte, a professor in Salk’s Gene Expression Laboratory and senior author of the paper. “Progeria is an ideal aging model because it allows us to devise an intervention, refine it and test it again quickly.”
With an early onset and fast progression, progeria is one of the most severe forms of a group of degenerative disorders caused by a mutation in the LMNA gene. Both mice and humans with progeria show many signs of aging, including DNA damage, cardiac dysfunction and dramatically shortened life span. The LMNA gene normally produces two similar proteins inside a cell: lamin A and lamin C. Progeria shifts the production of lamin A to progerin. Progerin is a shortened, toxic form of lamin A that accumulates with age and is exacerbated in those with progeria.