Scientists have uncovered how stem cells in the olfactory system continually regenerate neurons responsible for our sense of smell.

When injured, cells have well-regulated responses to promote healing. These include a long-studied self-destruction process that cleans up dead and damaged cells as well as a more recently identified phenomenon that helps older cells revert to what appears to be a younger state to help grow back healthy tissue.
Now, a new study in mice led by researchers at Washington University School of Medicine in St. Louis and the Baylor College of Medicine reveals a previously unknown cellular purging process that may help injured cells revert to a stem cell-like state more rapidly. The investigators dubbed this newly discovered response cathartocytosis, taking from Greek root words that mean cellular cleansing.
Published online in the journal Cell Reports, the study used a mouse model of stomach injury to provide new insights into how cells heal, or fail to heal, in response to damage, such as from an infection or inflammatory disease.
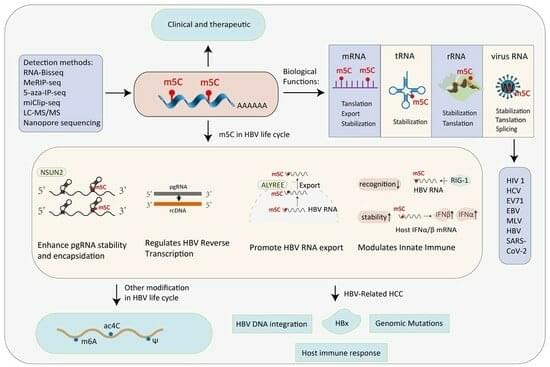
Hepatitis B virus (HBV) remains a major global health challenge, with over 296 million people chronically infected worldwide. Despite the availability of antiviral therapies, a functional cure is rarely achieved, highlighting the need for novel therapeutic strategies. RNA 5-methylcytosine (m5C) is a pivotal epitranscriptomic mark implicated in RNA stability, transport, and translation. Emerging evidence shows that m5C is conserved within HBV RNA and plays critical roles in the viral life cycle. This review provides a comprehensive overview of the molecular mechanisms governing m5C deposition and recognition, summarizes recent advances in m5C biology, and highlights the emerging role of epitranscriptomic m5C regulation in HBV infection.
Support this channel on Patreon to help me make this a full time job: https://www.patreon.com/whatdamath (Unreleased videos, extra footage, DMs, no ads)Alter…

Michal Smolski, his consultant urologist, said he was pleased to say “it all went as planned”
(Yes you can get this procedure simply by going to the UK. Trust me it’s cheaper than the USA)
A team of surgeons at a hospital trust are celebrating performing their 1,000th prostatectomy using robotic surgeries.
Lancashire Teaching Hospitals NHS Foundation Trust have been using the Da Vinci Xi robotic system for complete or partial prostate removals since 2017.
Surgeons at Chorley and South Ribble Hospital conducted the milestone surgery on patient James Goggin.


By boosting the activity of cellular ‘power stations’ in the brains of mice with a dementia-like condition, an international team of researchers has reversed pathological memory loss.
Problems with energy-producing cellular structures called mitochondria have previously been linked to neurodegenerative diseases such as Alzheimer’s. Before now, it wasn’t clear if this was a cause or a consequence of these conditions.
“This work is the first to establish a cause-and-effect link between mitochondrial dysfunction and symptoms related to neurodegenerative diseases, suggesting that impaired mitochondrial activity could be at the origin of the onset of neuronal degeneration,” says Giovanni Marsicano, a neuroscientist from the French National Institute of Health and Medical Research (INSERM).

For decades now, the commonly-accepted view among neuroscientists has been that following amputation of a limb, neighboring regions rearrange and essentially take over the area previously assigned to the now missing limb. This has relied on evidence from studies carried out after amputation, without comparing activity in the brain maps beforehand.
To investigate this contradiction, a team of researchers followed three individuals due to undergo amputation of one of their hands. This is the first time a study has looked at the hand and face maps of individuals both before and after amputation.
The researchers examined the signals from the pre-amputation finger maps and compared them against the maps post-amputation. Analysis of the ‘before’ and ‘after’ images revealed a remarkable consistency: even with their hand now missing, the corresponding brain region activated in an almost identical manner.
The study’s senior author, said: Because of our previous work, we suspected that the brain maps would be largely unchanged, but the extent to which the map of the missing limb remained intact was jaw-dropping.
To complement their findings, the researchers compared their case studies to 26 participants who had had upper limbs amputated, on average 23.5 years beforehand. These individuals showed similar brain representations of the hand and lips to those in their three case studies, suggesting long-term evidence for the stability of hand and lip representations despite amputation.
The brain holds a ‘map’ of the body that remains unchanged even after a limb has been amputated, contrary to the prevailing view that it rearranges itself to compensate for the loss, according to new research.


China initiated its first multi-center clinical trial for brain-computer interface technology in neurocritical care on Sunday, marking a significant expansion of BCI applications beyond the rehabilitation of motor and cognitive functions.
The trial, launched in Tianjin, aims to explore new therapeutic approaches for severe neurological conditions.
Led by the Haihe Laboratory of Brain-Computer Interaction and Human-Machine Integration at Tianjin University and Tianjin Huanhu Hospital, the project brings together leading medical institutions from Beijing, Tianjin, Henan province, and other regions.
“This initiative will pave the way for broader medical applications, offering Chinese technologies, standards, and protocols for precise management of neurocritical conditions,” said Liu Xiuyun, deputy director of the Haihe Laboratory.