The tumor in one mouse that was injected with human cancer cells completely disappeared.

Cyrus Biotechnology in Seattle and Broad Institute of MIT and Harvard launch collaboration to develop optimized CRISPR gene technology.
Cyrus Biotechnology, Inc. Lucas Nivon, 206−258−6561 [email protected]
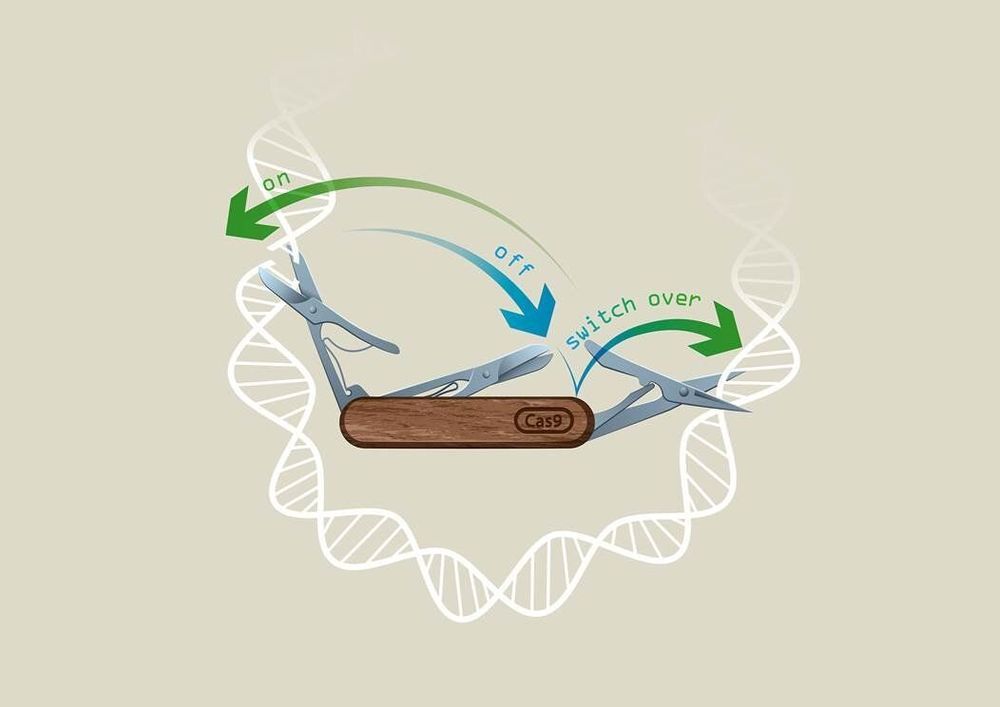
Researchers in Vienna from Ulrich Elling’s laboratory at IMBA—Institute of Molecular Biotechnology of the Austrian Academy of Sciences—in collaboration with the Vienna BioCenter Core Facilities have developed a revolutionary CRISPR technology called “CRISPR-Switch,” which enables unprecedented control of the CRISPR technique in both space and time.
CRISPR/Cas9 technology is based on a modified version of a bacterial defense system against bacteriophages. One of the landmark discoveries for this technique in fact was laid in Vienna and published in 2012 in a study co-authored by Emmanuelle Charpentier and VBC Ph.D. student, Krzysztof Chylinski. Due to its power to also edit mammalian genomes, CRISPR/Cas9 has rapidly established itself as the most employed gene editing method in laboratories across the world with huge potential to find its way to the clinics to cure rare disease. Just a week ago, the first success in the treatment of sickle cell anemia was announced.
To control the power of genome editing, several groups have worked on systems to control editing activity. Scientists from the lab of Ulrich Elling at IMBA were now able to gain unprecedented control over sgRNA activity, in a system termed “CRISPR-Switch.” The results are published in the renowned journal Nature Communications.

Researchers have long known that some genes can cause cancer when overactive, but exactly what happens inside the cell nucleus when the cancer grows has so far remained enigmatic. Now, researchers at Karolinska Institutet in Sweden have found a new mechanism that renders one canonical driver of cancer overactive. The findings, published in Nature Genetics, create conditions for brand new strategies to fight cancer.
One gene that is called MYC is central for normal cell growth. However, if the gene mutates and/or becomes overactive, it could lead to abnormal cell growth and cancer. It is previously known that so-called super-enhancers, large regions in the DNA that develop near cancer genes, could somehow make the MYC gene overactive.
The current study increases our understanding of how this process takes place by highlighting how environmental cues can conspire with the architecture of the cell nucleus to cause overexpression. With the help of new laboratory techniques and computer models, the researchers show how the activation of the pathway of the signal-molecule WNT charges the super-enhancer with proteins that lures the MYC gene to the cell nucleus pores. The pores are situated on the membrane of the cell nucleus and control the flow of information between the cell nucleus and the cytoplasm.

Obesity is an important issue.
The brain mechanism that enables us to maintain a constant body temperature may also be the key to rapid weight loss, a new study finds. In experiments involving mice that were given a calorie-restricted diet, scientists at Scripps Research discovered that blocking a brain receptor that normally regulates body heat resulted in significant weight reductions.
The findings will be further explored as a potential treatment approach for obesity, which the World Health Organization has called a global epidemic. Obesity affects virtually all age and socioeconomic groups—increasing risk for heart disease, stroke, diabetes, cancer and many other serious health conditions.
The new study, led by Scripps Research Professor Bruno Conti, Ph.D., appears in Current Biology.
Researcher Dr. Michael Lustgarten has recently published a compact and very readable review that focuses on the role of the gut microbiome and its influence on skeletal muscle mass.
The gut microbiome
The microbiome describes a varied community of bacteria, archaea, eukarya, and viruses that inhabit our gut. The four bacterial phyla of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria comprise 98% of the intestinal microbiome.
Hollie Fraser, founder of Books On The Move, worldwide book sharing movement, creative director and ideaXme literature, reading and writing ambassador interviews Matthew Newman, 2019 best Kindle book author.
Matthew S. Newman, is a 39 year old cancer survivor and best-selling author of Starting At The Finish Line. He is a fitness aficionado, and a financial services industry speaker. His mission is help the wider public and to encourage financial preparedness. He does this by telling his story of brain cancer survival and the financial lessons and wisdom he learnt as a result.
Matthew’s book was named a Best Kindle Unlimited Books for 2019. To this day, fitness is still a big part of his life. You can see him here working out shortly after brain surgery! https://www.youtube.com/watch?v=J2G-Sq7iOuM
He is a TedX Talk participant and currently traveling the country giving talks to financial services organizations, such as Transamerica, Primerica, Raymond James and more. https://matthewsnewman.com/speaking
For the text version of this interview please visit www.radioideaxme.com from week commencing 2 December 2019.
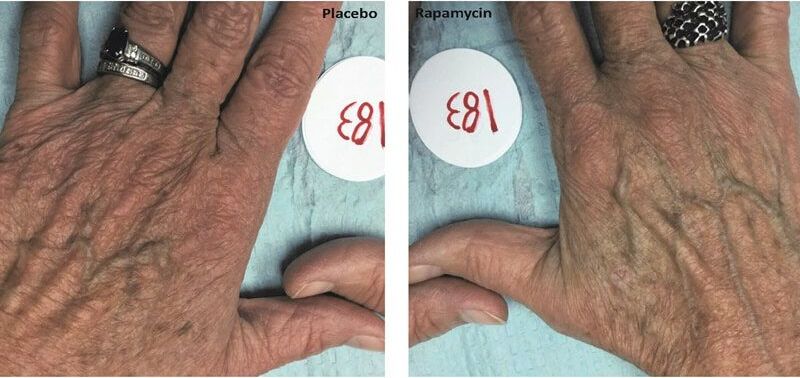
The search for youthfulness typically turns to lotions, supplements, serums and diets, but there may soon be a new option joining the fray. Rapamycin, a FDA-approved drug normally used to prevent organ rejection after transplant surgery, may also slow aging in human skin, according to a study from Drexel University College of Medicine researchers published in Geroscience.
Basic science studies have previously used the drug to slow aging in mice, flies, and worms, but the current study is the first to show an effect on aging in human tissue, specifically skin – in which signs of aging were reduced. Changes include decreases in wrinkles, reduced sagging and more even skin tone — when delivered topically to humans.
“As researchers continue to seek out the elusive ‘fountain of youth’ and ways to live longer, we’re seeing growing potential for use of this drug,” said senior author Christian Sell, PhD, an associate professor of Biochemistry and Molecular Biology at the College of Medicine. “So, we said, let’s try skin. It’s a complex organism with immune, nerve cells, stem cells – you can learn a lot about the biology of a drug and the aging process by looking at skin.”

Cyrus Biotechnology has teamed up with the Broad Institute to optimize CRISPR for use in humans. Feng Zhang, who had a hand in developing CRISPR, will serve as the Broad’s principal investigator for the collaboration.
One concern with using CRISPR-Cas9 to perform in vivo genome editing stems from the risk that the body will mount an immune response against the system. Those concerns have grown as researchers have shown that many people have antibodies against Cas9, reflecting the fact that the homologs of the protein used in genome editing systems are derived from bacteria that commonly infect people.
Cyrus, which lists Johnson & Johnson among its customers, thinks its technology can mitigate the risk of an immune reaction. That confidence reflects Cyrus’ experience of using software to identify and work around the epitopes in protein therapeutics that cause immunogenicity.