New images of the SARS-CoV-2 coronavirus demonstrate the potential of the helium ion microscopy in bioimaging, especially for the imaging of interactions between viruses and their host organisms.




“A famous neurologist Phil Kennedy made global headlines in the late 1990s for implanting wire electrodes in the brain of a ‘locked-in patient’ to control a computer cursor with their mind. Compared to Alexander Graham Bell in The Washington Post, Kennedy became known as ‘The Father of the Cyborgs’. Travelling to South America in 2014, he made further headlines when tiny electrodes were implanted inside his brain in order to continue his research. This film examines the ethical quandaries of self-experimentation and a future where technology and human brains combine.”
Screen Ireland/Fís Éireann is the development agency for the Irish Film Industry investing in talent, creativity and enterprise.

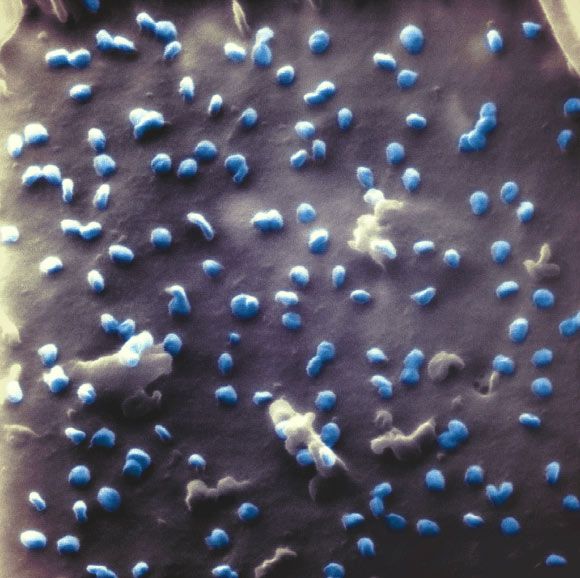
By blocking a receptor in macrophages, researchers were able to reverse aging in mice.
There are many suggested causes of old age. Telomere shortening, DNA damage, and depletion of stem cells are just a few of the proposed sources.
Recently, researchers found that a type of cell called a macrophage also plays a crucial role in aging. Macrophages are phagocytotic immune cells; they consume cells and other pathogens flagged by the immune system as dangerous. When macrophages need to consume a pathogen, their energy needs drastically go up.
Remember in biology when you were told that the mitochondria was the “powerhouse of the cell?” Cells produce energy through two main ways: glycolysis and oxidative phosphorylation. Both of these processes work inside the mitochondria by converting glucose into ATP, a molecule that acts cell’s “currency” on energy. Glycolysis converts glucose to ATP by degrading glucose into pyruvate. This reaction triggers the production of 2 ATP per molecule of glucose, which allows the cell can then use for other biological functions. Oxidative phosphorylation, on the other hand, is far more complicated, but also yields much more ATP. In a nutshell, it strips the electrons from hydrogen molecules so it can create an electrical gradient and produce up to 38 ATP per glucose molecule. Researchers found that as macrophages age, they tend to shut down these “metabolic pathways,” as they are called.

The “Flesh Without Blood” singer put a timeline on the plans, stating: “Let’s aim for chips by 2022. It’s experimental surgery but if it succeeds we’ll have the knowledge of the Gods haha.”
Lil Uzi agreed, writing: “Okay!!! I will call u for more detail.”
This is the FOURTH PART of the interview with Harold Katcher in Modern Healthspan YouTube channel.
Dr. Harold Katcher is a professor of Biology at the University of Maryland. He has been a pioneer in the field of cancer research, in the development of modern aspects of gene hunting and sequencing. He carries expertise in bioinformatics, chronobiology, and biotechnology. Dr. Katcher is currently working in the capacity of Chief Technical Officer at Nugenics Research exploring rejuvenation treatments in mammals.
In May 2020 there was a paper published on biorxiv about the rejuvenation of rats by over 50%. We did a review of the paper which you can find linked to above. In this interview series we talk with Dr. Harold Katcher, one of the main authors of the paper about the experiment, the steps to get validation, commercialization and how the results fit into his theories of aging.
In this video Dr. Katcher explains his theories on what causes aging, which he believes is a process that is programmed into us and is therefore malleable. I find his theories very interesting and compelling.
Dr. Katcher’s 2015 paper on the theory of aging is here:
Towards an evidence-based model of aging.
https://pubmed.ncbi.nlm.nih.gov/26054348/
The paper on plasma exchange can be found here https://www.biorxiv.org/content/10.1101/2020.05.07.082917v1.full.
