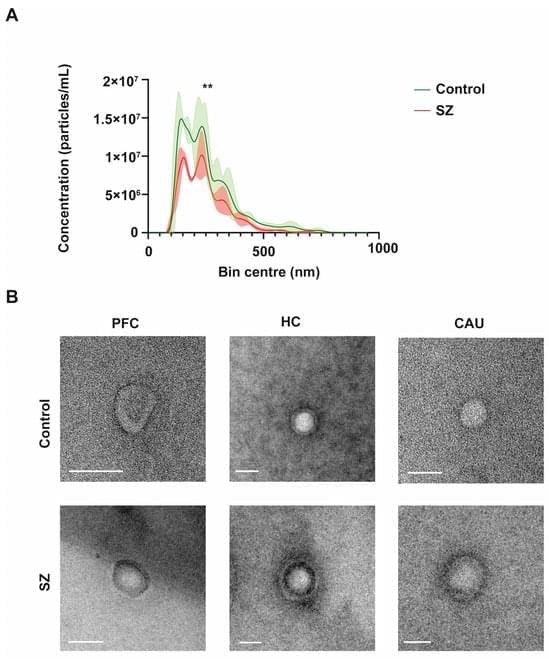
Extracellular vesicles (EVs) are tiny membranous structures that mediate intercellular communication. The role(s) of these vesicles have been widely investigated in the context of neurological diseases; however, their potential implications in the neuropathology subjacent to human psychiatric disorders remain mostly unknown. Here, by using next-generation discovery-driven proteomics, we investigate the potential role(s) of brain EVs (bEVs) in schizophrenia (SZ) by analyzing these vesicles from the three post-mortem anatomical brain regions: the prefrontal cortex (PFC), hippocampus (HC), and caudate (CAU). The results obtained indicate that bEVs from SZ-affected brains contain region-specific proteins that are associated with abnormal GABAergic and glutamatergic transmission.