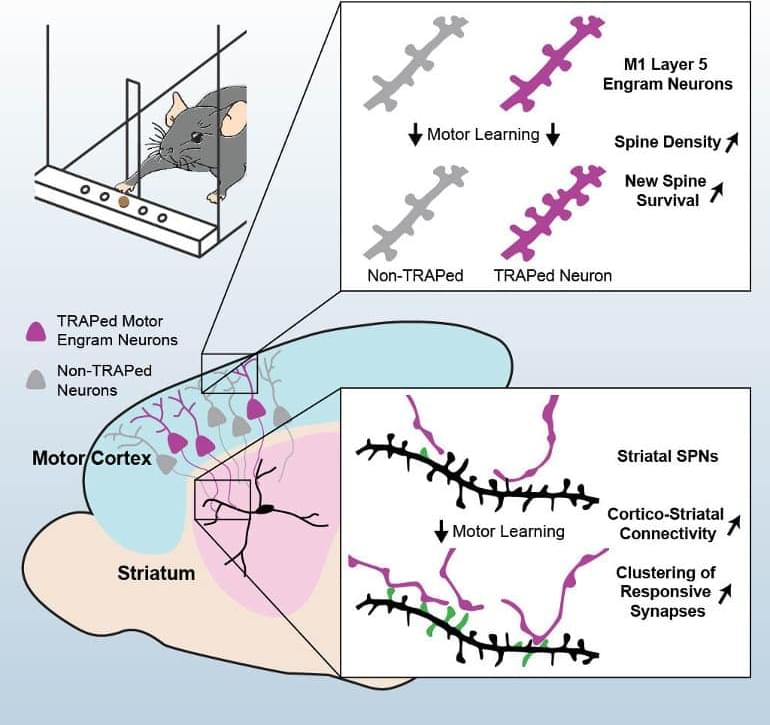
Summary: Study reveals how motor memories are formed and how they remain persistent. The findings may help illuminate the root cause of motor disorders like Parkinson’s disease.
Source: Stanford.
Why is it that someone who hasn’t ridden a bicycle in decades can likely jump on and ride away without a wobble, but could probably not recall more than a name or two from their 3rd grade class?