Category: biotech/medical – Page 1,648

Bioelectronics will be commonly used by 2025
Bioelectronics are a relatively new scientific field that could one day result in a new class of medicines that would not be pills or injections but miniaturised, implantable devices.
GSK believes that these devices could be programmed to read and correct the electrical signals that pass along the nerves of the body, including irregular or altered impulses that can occur in association with a broad range of diseases. The hope is that through these devices, disorders as diverse as inflammatory bowel disease, arthritis, asthma, hypertension and diabetes could be treated.
How electricity could replace your medications
Neurosurgeon and immunologist Kevin Tracey shares the frontiers of a new, hybrid field — bioelectronic medicine.
What Life Will be Like with Bioelectronic Medicine
In a first-of-its-kind gathering at the New York Academy of Sciences, researchers from some of the world’s leading universities and institutions convened to discuss at the 13th annual Key Symposium the various applications of bioelectronic medicine, the cutting-edge field that uses technology to treat disease and injury. While still in early stages of development, bioelectronic medicine has already been proven in studies and clinical trials to successfully treat conditions including paralysis and rheumatoid arthritis.
This panel, moderated by Miles O’Brien from PBS’ NewsHour, discusses what life will be like when we can fully modulate the nervous system and the impact that would have on disease, drugs, the healthcare industry, personal freedom, and privacy. The panel includes Polina Anikeeva, PhD, from the Massachusetts Institute of Technology, Chad Bouton from The Feinstein Institute for Medical Research at Northwell Health, Peder S. Olofsson, MD, PhD, from the Karolinska Institutet, and Doug Weber, PhD, from the U.S. Defense Advanced Research Projects Administration.
To learn more about this year’s event, visit feinsteininstitute.org/key-symposium

New DNA repair-kit successfully fixes hereditary disease in patient-derived cells
Genetic mutations which cause a debilitating hereditary kidney disease affecting children and young adults have been fixed in patient-derived kidney cells using a potentially game-changing DNA repair-kit. The advance, developed by University of Bristol scientists, is published in Nucleic Acids Research.
In this new study, the international team describe how they created a DNA repair vehicle to genetically fix faulty podocin, a common genetic cause of inheritable Steroid Resistant Nephrotic Syndrome (SRNS).
Podocin is a protein normally located on the surface of specialized kidney cells and essential for kidney function. Faulty podocin, however, remains stuck inside the cell and never makes it to the surface, terminally damaging the podocytes. Since the disease cannot be cured with medications, gene therapy which repairs the genetic mutations causing the faulty podocin offers hope for patients.
How we can finally win the fight against aging | Aubrey De Grey | TEDxMünchen
For more information on Aubrey de Grey, please visit our website www.tedxmuenchen.de.
Dr. Aubrey de Grey is a biomedical gerontologist based Mountain View, California, USA, and is the Chief Science Officer of SENS Research Foundation, a California-based 501©(3) biomedical research charity that performs and funds laboratory research dedicated to combating the aging process. He is also Editor-in-Chief of Rejuvenation Research, the world’s highest-impact peer-reviewed journal focused on intervention in aging. He received his BA in computer science and Ph.D. in biology from the University of Cambridge. His research interests encompass the characterisation of all the accumulating and eventually pathogenic molecular and cellular side-effects of metabolism (“damage”) that constitute mammalian aging and the design of interventions to repair and/or obviate that damage.
Twitter: @aubreydegrey.
This talk was given at a TEDx event using the TED conference format but independently organized by a local community.

Locusts can detect cancer in humans
A new study led by Michigan State University (MSU) has found that locusts can reliably detect through smell a variety of human cancers. The insects can not only “smell” the difference between healthy and cancerous cells, but they can also distinguish between different cancer cell lines. These findings could provide a basis for devices which use locust sensory neurons to enable the early detection of cancer by using only biomarkers in a patient’s breath.
“Noses are still state of the art,” said study senior author Debajit Saha, an assistant professor of Biomedical Engineering at MSU. “There’s really nothing like them when it comes to gas sensing. People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly.”
Cancer cells function differently from healthy ones, and create different chemical compounds as they grow. If these chemicals reach the lungs or airways – which happens in most types of cancer – they can be detected in exhaled breath. “Theoretically, you could breathe into a device, and it would be able to detect and differentiate multiple cancer types and even which stage the disease is in. However, such a device isn’t yet close to being used in a clinical setting,” Professor Saha explained.
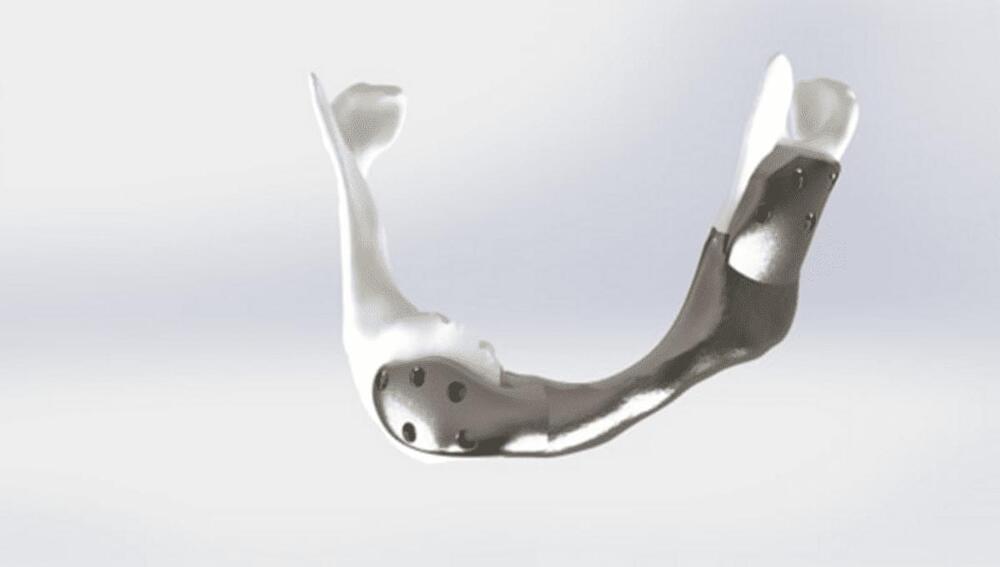
Cancer Patient Receives World First 3D Printed Titanium Jaw
The jawbone was reconstructed from the patient’s 3D MRI and CT scans, making it a perfect fit in its new boney home.
This is a major step in the treatment of head and neck cancer, a disease which affects as many as 600,000 people every year. For most people, especially those whose cancer is caught early, the treatment is fairly minimal surgery, sometimes using lasers, or radiation therapy.
For some, though, more aggressive action is needed, and part of the lower jaw must be removed. As you might expect, this can have a massive impact on the patient’s life, since it renders them unable to speak, chew, and other essential actions – not to mention being quite noticeable from an aesthetic viewpoint.

Scientists Create First Synthetic Embryo, Allow It to Develop a Functioning Brain and Organs
The frightening future implications of new report from researchers at the Weizmann Institute of Science in Israel caused a stir among observers of the international science community.
Using neither sperm nor egg, researchers created the world’s first synthetic mouse embryo and watched it grow for over eight days inside of a specially designed bioreactor that served as a womb, according to Live Science Magazine.
The article describes what occurs inside the artificial womb. “Within the device, embryos float in small beakers of nutrient-filled solution, and the beakers are all locked into a spinning cylinder that keeps them in constant motion. This movement simulates how blood and nutrients flow to the placenta. The device also replicates the atmospheric pressure of a mouse uterus.”
