What can the decline of the Roman Empire and the end of European feudalism tell us about COVID-19 and the future of the West?


Circa 2010
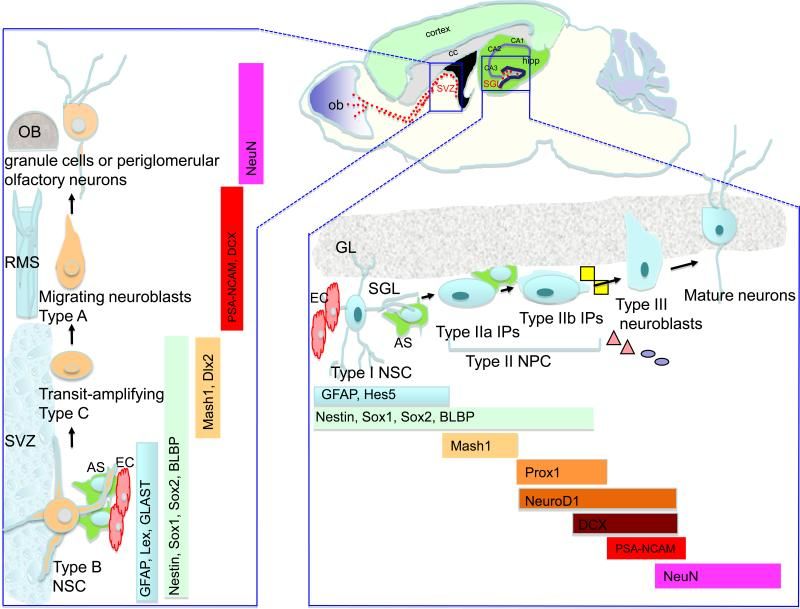
In this review, we consider the evidence that a reduction in neurogenesis underlies aging-related cognitive deficits, and impairments in disorders such as Alzheimer’s disease (AD). The molecular and cellular alterations associated with impaired neurogenesis in the aging brain are discussed. Dysfunction of presenilin-1, misprocessing of amyloid precursor protein and toxic effects of hyperphosphorylated tau and β-amyloid likely contribute to impaired neurogenesis in AD. Since factors such as exercise, enrichment and dietary energy restriction enhance neurogenesis, and protect against age-related cognitive decline and AD, knowledge of the underlying neurogenic signaling pathways could lead to novel therapeutic strategies for preserving brain function. In addition, manipulation of endogenous neural stem cells and stem cell transplantation, as stand-alone or adjunct treatments, seem promising.
There is a progressive decline in the regenerative capacity of most organs with increasing age, resulting in functional decline and poor repair from injury and disease. Once thought to exist only in high turnover tissues, such as the intestinal lining or bone marrow, it now appears that most tissues harbor stem cells that contribute to tissue integrity throughout life. In many cases, stem cell numbers decrease with age, suggesting stem cell aging may be of fundamental importance to the biology of aging (for review, see Ref. [1]). Therefore, understanding the regulation of stem cell maintenance and/or activation is of considerable relevance to understanding the age-related decline in maintaining tissue integrity, function, and regenerative response.
The adult brain contains neural stem cells (NSCs) that self-renew, proliferate and give rise to neural progenitor cells (NPC) that exhibit partial lineage-commitment. Following several cycles of proliferation, NPC differentiate into new neurons and glia. NSCs are increasingly acknowledged to be of functional significance and harbor potential for repair of the diseased or injured brain. The dramatic decline in neurogenesis with age is thought to underlie impairments in learning and memory, at least in part. Aging is also the greatest risk factor for Alzheimer’s disease (AD), a neurodegenerative disease characterized by progressive loss of memory and cognitive decline. Alterations in neurogenesis have been described extensively in animal models of AD, and key proteins involved in AD pathogenesis are shown to regulate neurogenesis.

Breast cancer is the leading cause of cancer death in women around the world, responsible for 1700 deaths every day. Although the vast majority of breast cancers are treatable, the most aggressive subtype—triple negative breast cancer (TNBC) – has a high recurrence rate, a high potential for metastasis and shows resistance to conventional treatments, leading to very poor prognosis and survival outcomes. A team of researchers at the Research Institute of the McGill University Health Center (RI-MUHC) conducted a preclinical study and discovered a novel targeted combination therapy that efficiently reduced tumor growth in metastatic breast cancer. Published in Nature Communications, their findings could lead to the development of a novel first line targeted therapy for the treatment of TNBC, with the prospect of rapidly transitioning to clinical trials in humans.
“There is no targeted therapy for TNBC. Chemotherapy treatment can even enrich these tumors in cancer stem cells and be detrimental to the patient, as we have shown in a previous study,” says Dr. Jean-Jacques Lebrun, senior scientist in the Cancer Research Program at the RI-MUHC and principal investigator of the study. “Filling that huge medical gap was our motivation in conducting this study.”
While most breast cancers have one of three main receptors that are like entrance gates for treatments—estrogen, progesterone and a protein called human epidermal growth factor (HER2) – TNBC has none, thus the name triple negative breast cancer. Using state-of-the-art technologies such as gene editing and genome-wide molecular approaches, the team identified two pathways which could be targeted in a therapeutic strategy.
“Once the virus is recognised, the CRISPR enzyme is activated and chops up the virus,” she said.
Paris (AFP)
Scientists have used CRISPR gene-editing technology to successfully block the transmission of the SARS-CoV-2 virus in infected human cells, according to research released Tuesday that could pave the way for Covid-19 treatments.
Writing in the journal Nature Communications, researchers in Australia said the tool was effective against viral transmissions in lab tests, adding that they hoped to begin animal trials soon.
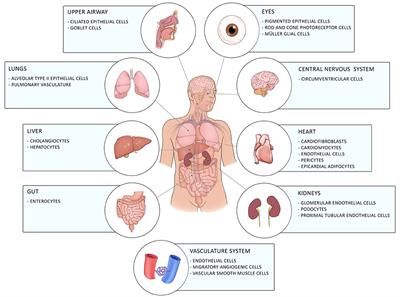
Coronavirus disease-19 caused by the novel RNA betacoronavirus SARS-CoV2 has first emerged in Wuhan, China in December 2019, and since then developed into a worldwide pandemic with 99 million people afflicted and 2.1 million fatal outcomes as of 24th January 2021. SARS-CoV2 targets the lower respiratory tract system leading to pneumonia with fever, cough, and dyspnea. Most patients develop only mild symptoms. However, a certain percentage develop severe symptoms with dyspnea, hypoxia, and lung involvement which can further progress to a critical stage where respiratory support due to respiratory failure is required. Most of the COVID-19 symptoms are related to hyperinflammation as seen in cytokine release syndrome and it is believed that fatalities are due to a COVID-19 related cytokine storm. Treatments with anti-inflammatory or anti-viral drugs are still in clinical trials or could not reduce mortality. This makes it necessary to develop novel anti-inflammatory therapies. Recently, the therapeutic potential of phytocannabinoids, the unique active compounds of the cannabis plant, has been discovered in the area of immunology. Phytocannabinoids are a group of terpenophenolic compounds which biological functions are conveyed by their interactions with the endocannabinoid system in humans. Here, we explore the anti-inflammatory function of cannabinoids in relation to inflammatory events that happen during severe COVID-19 disease, and how cannabinoids might help to prevent the progression from mild to severe disease.
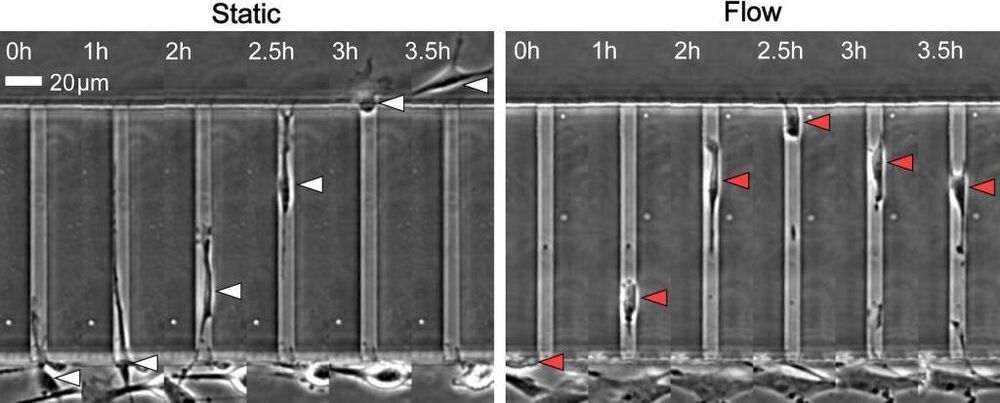
Researchers have identified a specialized protein that appears to help prevent tumor cells from entering the bloodstream and spreading to other parts of the body.
“We have discovered that this protein, TRPM7, senses the pressure of fluid flowing in the circulation and stops the cells from spreading through the vascular system,” said Kaustav Bera, a Image 1: Overexpressing protein TRPM7 in cancer cells greatly reduces entry into the blood vessels. Image 2: In static conditions, cells enter microchannels, whereas 40-60% reverse direction when fluid is flowing. Courtesy of Johns Hopkins University.
Johns Hopkins University news releases are available online, as is information for reporters. To arrange an interview with a Johns Hopkins expert, contact a media representative listed above. Find more Johns Hopkins experts on the Experts Hub, and more Johns Hopkins stories on the Hub.

A warehouse in frigid Minnesota is being transformed into a new kind of homeless shelter, one where residents aren’t just given a bed, but their own tiny home.
This new tiny house village is designed to overcome the limitations of traditional homeless shelters, while also addressing the unique problems of sheltering people during a pandemic.
“These new data are encouraging and reinforce our belief that the Moderna COVID-19 Vaccine should remain protective against newly detected variants,” Stéphane Bancel, Moderna’s chief executive officer, said in a statement. “These findings highlight the importance of continuing to vaccinate populations with an effective primary series vaccine.”
The company also said it is developing a booster candidate: a 50–50 mix of its currently authorized COVID-19 vaccine and another messenger RNA vaccine it has developed.
The delta variant is the fast-moving form of the coronavirus that is now found in 96 countries, including the United States.

Many people with rheumatoid arthritis, or RA, report having trouble thinking clearly, problems with memory, and difficulty concentrating.
These symptoms, known as brain fog, are widespread in people with chronic inflammatory conditions, including RA, Sjogren’s syndrome, and multiple sclerosis.

If you want to learn, then you have to break some things.
Summary: Brain cells snap DNA in more places and in more cell types than previously realized in order to express genes for learning and memory.
Source: Picower Institute for Learning and Memory
The urgency to remember a dangerous experience requires the brain to make a series of potentially dangerous moves: Neurons and other brain cells snap open their DNA in numerous locations—more than previously realized, according to a new study—to provide quick access to genetic instructions for the mechanisms of memory storage.
The extent of these DNA double-strand breaks (DSBs) in multiple key brain regions is surprising and concerning, said study senior author Li-Huei Tsai, Picower Professor of Neuroscience at MIT and director of The Picower Institute for Learning and Memory, because while the breaks are routinely repaired, that process may become more flawed and fragile with age. Tsai’s lab has shown that lingering DSBs are associated with neurodegeneration and cognitive decline and that repair mechanisms can falter.