The new Covid pills from Merck and Pfizer offer a ton of promise. But there’s plenty we still don’t know about how well they work.

The last year has been a story of triumph in the vaccine world, with the rapid development of two highly successful vaccines for Covid-19, one developed by Moderna and the other by Pfizer and BioNTech. Now that flu season is approaching, why are we still using 50-year-old technology for the flu vaccine?
The reason the Covid-19 vaccines were developed so quickly is that they used a new, much faster and easier-to-create type of vaccine technology, based on messenger RNA, or mRNA. What’s even more exciting is that we now have an overwhelming amount of evidence, from real-world experience, that these vaccines are remarkably safe and effective.
Now, anti-vaxxers and the “vaccine hesitant” are claiming they don’t trust the vaccine because it was developed too fast. That’s ridiculous: the real reason they don’t trust the vaccine is because they’re consuming a steady diet of anti-vaccine nonsense, promoted by a combination of right-wing media and the Disinformation Dozen (who include left-wing as well as right-wing zealots). But let’s not go down that rabbit hole today.
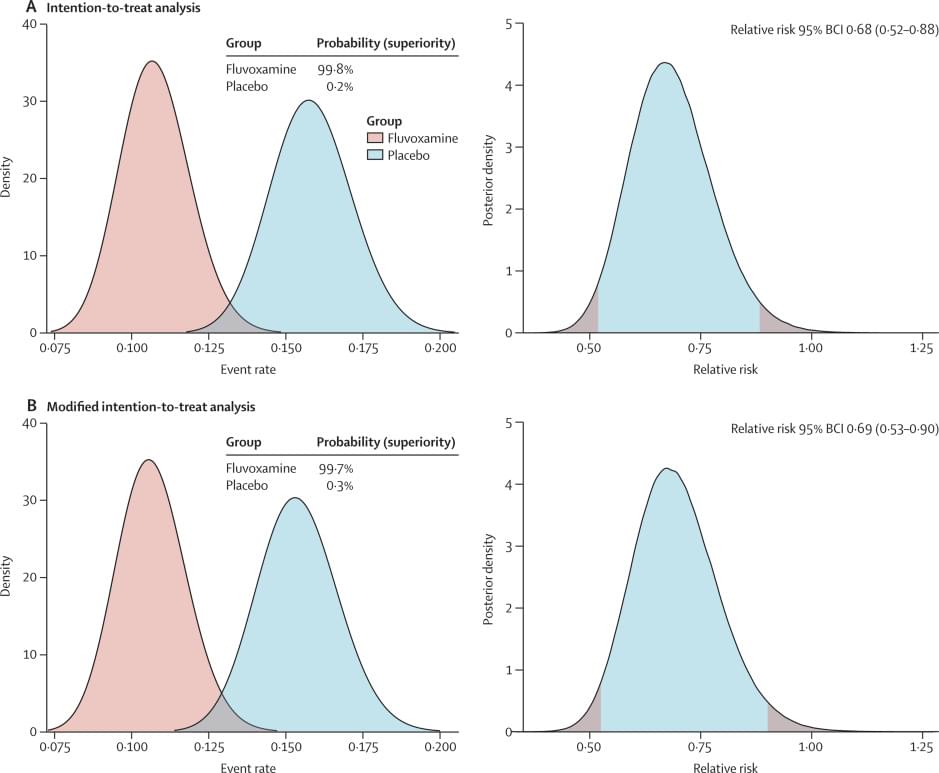
I may have already posted about this, but this is more data from The Lancet.
Background.
Recent evidence indicates a potential therapeutic role of fluvoxamine for COVID-19. In the TOGETHER trial for acutely symptomatic patients with COVID-19, we aimed to assess the efficacy of fluvoxamine versus placebo in preventing hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to a tertiary hospital due to COVID-19.
Methods.
This placebo-controlled, randomised, adaptive platform trial done among high-risk symptomatic Brazilian adults confirmed positive for SARS-CoV-2 included eligible patients from 11 clinical sites in Brazil with a known risk factor for progression to severe disease. Patients were randomly assigned (1:1) to either fluvoxamine (100 mg twice daily for 10 days) or placebo (or other treatment groups not reported here). The trial team, site staff, and patients were masked to treatment allocation. Our primary outcome was a composite endpoint of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 up to 28 days post-random assignment on the basis of intention to treat. Modified intention to treat explored patients receiving at least 24 h of treatment before a primary outcome event and per-protocol analysis explored patients with a high level adherence (80%). We used a Bayesian analytic framework to establish the effects along with probability of success of intervention compared with placebo. The trial is registered at ClinicalTrials dot gov (NCT04727424) and is ongoing.
Treatment with fluvoxamine (100 mg twice daily for 10 days) among high-risk outpatients with early diagnosed COVID-19 reduced the need for hospitalisation defined as retention in a COVID-19 emergency setting or transfer to a tertiary hospital.
Creating a world where no woman has to die giving life — temitayo erogbogbo, global advocacy director, MSD for mothers, merck sharp & dohme.
Mr. Temitayo (Tayo) Erogbogbo, is Global Advocacy Director of MSD for Mothers (https://www.msdformothers.com/), at Merck Sharp & Dohme.
Tayo has two decades of combined private sector and international development experience, 13 years of which was spent in the pharmaceutical industry in multiple roles across community relations, government affairs, marketing and sales.
As the Global Advocacy Director of MSD for Mothers, Tayo is responsible for global and national strategic partnerships and programs to bring about policies and practice changes to improve maternal health care, and strengthen health systems, particularly where private sector approaches can be leveraged for greater impact.
Prior to MSD for Mothers, Tayo led the establishment of an adolescents and youth constituency at The Partnership for Maternal, Newborn and Child Health (PMNCH), a multi-constituency partnership hosted by the World Health Organization, to advocate for better sexual, reproductive, maternal, newborn, child, and adolescent health policies and services at global, regional, and national levels. Additionally, he contributed to the development of the Global Strategy for Women’s, Children’s, and Adolescents’ Health 2016–2030.


In an unprecedented atlas, researchers begin to map how genes are turned on or off in different cells, a step toward better understanding the connections between genetics and disease.
Researchers at University of California San Diego have produced a single-cell chromatin atlas for the human genome. Chromatin is a complex of DNA
DNA, or deoxyribonucleic acid, is a molecule composed of two long strands of nucleotides that coil around each other to form a double helix. It is the hereditary material in humans and almost all other organisms that carries genetic instructions for development, functioning, growth, and reproduction. Nearly every cell in a person’s body has the same DNA. Most DNA is located in the cell nucleus (where it is called nuclear DNA), but a small amount of DNA can also be found in the mitochondria (where it is called mitochondrial DNA or mtDNA).
And it can be hacked.
The authors of a new study in Nature Catalysis reprogrammed these blobs—called exosomes—into an army of living nanobioreactors. It’s a seemingly simple process of mix and match: each blob is filled with a different chemical that’s involved in a biological reaction. By bringing two together, the blobs merge into a single squishy container, allowing the two chemicals to react.
The results were explosive. The tiny bioreactors pumped out energy molecules, called ATP, inside living cells. The burst of energy saved injured cells, providing them with a boost of power to fight back against dangerous molecules that otherwise lead to cell death.

Great video of vital biomolecular processes.
COVID-19 mRNA vaccines deliver directions to make a protein that educates our immune system, so it will neutralize the virus in future encounters. The mRNA-containing lipid particles are taken up by specialized immune system cells. See more: COVIDVaccineAnswers.org
Animation created by and for the Vaccine Makers Project.
Copyright © 2021, Medical History Pictures, Inc. All rights reserved.
The Vaccine Makers Project (VMP) is the classroom-based program of the Vaccine Education Center at the Children’s Hospital of Philadelphia (VEC at CHOP). The Center’s team is composed of scientists, physicians, mothers and fathers devoted to the study and prevention of infectious diseases. The Center was launched in October 2000 to provide accurate, comprehensive and up-to-date information about vaccines and the diseases they prevent. The VMP program is committed to public education about vaccine science via scientifically supported, historically accurate, and emotionally compelling content.

In one of the mysteries of mammalian development, every cell in the early female embryo shuts down one of its two copies of the X chromosome, leaving just one functional. For years, the mechanics behind this X chromosome inactivation have been murky, but scientists from the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA have now taken a major step forward in understanding the process.
Their findings, based on research on mouse stem cells, upend previous assumptions about how X inactivation is initiated in female embryos and could lead to new ways to treat some genetic disorders, as well as a better understanding of how genes on other chromosomes are silenced.
“X inactivation is one of the most fundamentally important processes in development, and I think this study is a slam dunk in finally understanding it,” said Kathrin Plath, a professor of biological chemistry and senior author of the paper, published in the journal Cell.