😗
More AI in the exam room means doctors can spend more time actually talking to patients.
Circa 2019 😀
Because they can process massive amounts of data, computers can perform analytical tasks that are beyond human capability. Google, for instance, is using its computing power to develop AI algorithms that construct two-dimensional CT images of lungs into a three-dimensional lung and look at the entire structure to determine whether cancer is present. Radiologists, in contrast, have to look at these images individually and attempt to reconstruct them in their heads. Another Google algorithm can do something radiologists cannot do at all: determine patients’ risk of cardiovascular disease by looking at a scan of their retinas, picking up on subtle changes related to blood pressure, cholesterol, smoking history and aging. “There’s potential signal there beyond what was known before,” says Google product manager Daniel Tse.
The Black Box Problem
AI programs could end up revealing entirely new links between biological features and patient outcomes. A 2019 paper in JAMA Network Open described a deep-learning algorithm trained on more than 85,000 chest x-rays from people enrolled in two large clinical trials that had tracked them for more than 12 years. The algorithm scored each patient’s risk of dying during this period. The researchers found that 53 percent of the people the AI put into a high-risk category died within 12 years, as opposed to 4 percent in the low-risk category. The algorithm did not have information on who died or on the cause of death. The lead investigator, radiologist Michael Lu of Massachusetts General Hospital, says that the algorithm could be a helpful tool for assessing patient health if combined with a physician’s assessment and other data such as genetics.
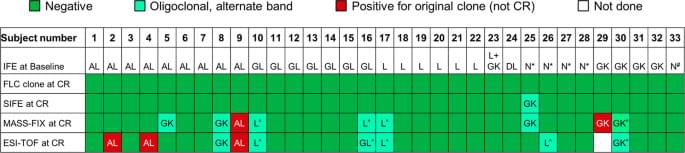
The Mayo Foundation Institutional Review Board (IRB) approved the study. All patients gave written informed consent to have their medical records reviewed and samples analyzed according to IRB requirements and federal regulations. Patients were eligible for this retrospective study if they: were diagnosed with AL amyloidosis between January 2000 and May 2015; were classified as amyloidosis complete hematologic response by immunofixation electrophoresis (IFE), serum free light chain (FLC) by consensus criteria;6,7 had a negative bone marrow by six-color flow cytometry; and had both a stored research sample prior to starting a line of therapy and a repeat sample while in complete hematologic response. The diagnosis of amyloidosis was made by Congo red with green birefringence under polarized light; the typing of the amyloid was with immunohistochemical stains or proteomics8,9. Supplementary Figure 1 is a consort diagram illustrating patient selection. Median time from institution of therapy to complete response (CR) sample was 18 months (interquartile range 9.1, 20 months).
The serum IFE (SIFE), urine IFE (UIFE), FLC, and bone marrow measurements were done as part of routine clinical practice as previously described4,5. Urine samples were concentrated to a maximum of 200× to achieve final concentrations of urine protein between 20 and 80 g/L4,5. The FLC assay (Freelite™, The Binding Site Ltd.) was performed on a Siemens BNII nephelometer10, and an abnormal FLC result was defined as an abnormal FLC κ/λ ratio. Bone marrow clonality was determined by six-color flow cytometry11. This method has sensitivity of ~10−4 to 10−5.
For MASS-FIX, immunoglobulins were enriched from serum using camelid-derived nanobodies directed against the heavy-chain constant domains of IgG, IgA, and IgM or the light-chain constant domains of κ and λ (Thermo Fisher Scientific)4,5. The +1 and +2 charge states of the light chains and heavy chains were measured by configuring the mass spectrometer to analyze ions between an m/z of 9000–32,000 Da.

Glucose monitoring with mass spectrometry circa 2013.
Diabetes is a common endocrine disorder characterized by hyperglycemia leading to nonenzymatic glycation of proteins, responsible for chronic complications. The development of mass spectrometric techniques able to give highly specific and reliable results in proteome field is of wide interest for physicians, giving them new tools to monitor the disease progression and the possible complications related to diabetes, as well as the effectiveness of therapeutic treatments. This paper reports and discusses some of the data pertaining protein glycation in diabetic subjects obtained by matrix-assisted laser desorption ionization (MALDI) mass spectrometry (MS). The preliminary studies carried out by in vitro protein glycation experiments show clear differences in molecular weight of glycated and unglycated proteins. Then, the attention was focused on plasma proteins human serum albumin (HSA) and immunoglobulin G (IgG). Enzymatic degradation products of in vitro glycated HSA were studied in order to simulate the in vivo enzymatic digestion of glycated species by the immunological system leading to the highly reactive advanced glycation end-products (AGEs) peptides. Further studies led to the evaluation of glycated Apo A-I and glycated haemoglobin levels. A different MALDI approach was employed for the identification of markers of disease in urine samples of healthy, diabetic, nephropathic, and diabetic-nephropathic subjects.
Diabetes is usually considered as a disease related to glucose dysmetabolism. In particular, type 1 diabetes is a chronic disease related to metabolism of carbohydrates, fats, and proteins, caused by the lack of insulin. It results from the marked and progressive inability of the pancreas to secrete insulin, due to autoimmune destruction of the beta cells. On the other hand, type 2 diabetes is caused by islet beta cells being unable to secrete adequate insulin in response to varying degrees of overnutrition, inactivity, obesity, and insulin resistance. Nowadays, the burden of diabetes is enormous, due to its increasing global prevalence and the occurrence of chronic complications affecting many tissues (retinopathy, nephropathy, neuropathy, and cardiovascular disease) reflecting in high direct and indirect costs [1].
This view may be seen somehow reductive, considering that the side effects of the previous mechanisms are at systemic level, and, taking into account the high complexity of the biological environment, it necessarily reflects on a high number of different pathological pathways, catalyzed by the glucose dysmetabolism. In this context, considering the Maillard reaction pattern [2], proteins seem to be at first sight the target of the glucose molecules circulating at high level in diabetes, and only some papers gave contradictory results about the reactivity of sugar with respect to DNA [3, 4].

I’m convinced a lot of diseases, MS, parkinsons, alzheimers, most cancers, are the result of bacterial or viral infections.
Building on a growing body of evidence linking viral infections with neurodegenerative disease, a new study published in Nature Communications has demonstrated how certain molecules on the surfaces of viruses can promote the aggregation of toxic proteins associated with diseases such as Alzheimer’s and Parkinson’s.
The idea that microbial infections can trigger neurodegenerative disease is not new. As far back as the 1950s scientists have been postulating ways an acute viral infection can lead to progressive neurological problems years, or even decades, later.
While evidence for this association is certainly growing, the mechanisms by which viral threats can influence the progression of brain diseases are still resolutely hypothetical. A common hypothesis speculates some viral infections may trigger abnormal immune responses that subsequently linger for years, ultimately generating neurological damage associated with some brain diseases.
I am pleased to share our most recent video on the “Dementia Safety Study” funded by Maximum Life Foundation and run by IHS, in which BioViva did the data analysis. This treatment shows promise for the millions of people who have dementia today. Though not a cure, it is a step in the right direction.
Can gene therapy delay or reverse Alzheimer’s and other dementias?
Want to learn more about BioViva? Visit: https://bioviva-science.com/
Interested in aging tests? https://bioviva-science.com/collections/products.
Facebook https://www.facebook.com/BiovivaScience.
Twitter https://twitter.com/BioVivaScience.
Instagram https://www.instagram.com/biovivascience.
LinkedIn https://www.linkedin.com/in/liz-parrish.

The strategy outlines how AI can be applied to defence and security in a protected and ethical way. As such, it sets standards of responsible use of AI technologies, in accordance with international law and NATO’s values. It also addresses the threats posed by the use of AI by adversaries and how to establish trusted cooperation with the innovation community on AI.
Artificial Intelligence is one of the seven technological areas which NATO Allies have prioritized for their relevance to defence and security. These include quantum-enabled technologies, data and computing, autonomy, biotechnology and human enhancements, hypersonic technologies, and space. Of all these dual-use technologies, Artificial Intelligence is known to be the most pervasive, especially when combined with others like big data, autonomy, or biotechnology. To address this complex challenge, NATO Defence Ministers also approved NATO’s first policy on data exploitation.
Individual strategies will be developed for all priority areas, following the same ethical approach as that adopted for Artificial Intelligence.

Taking psilocybin can affect one’s emotional state when listening to music, according to new research presented earlier this month at the 34th ECNP Congress in Lisbon.
Psilocybin, the active psychedelic component of magic mushrooms, has previously shown great promise when used in therapy settings for the treatment of depression. Many of these clinical trials often make use of selected music playlists to support the subjective psychedelic experience felt by the trial participant.
Now, scientists believe that this action of combining psilocybin with music may result in enhanced emotional processing on behalf of the participant, implying that music should be treated as a more active component of psilocybin therapy.
Collaboration, transparency & urgency for rare disease research — mike graglia, managing director & co-founder, syngap research fund — SRF.
Mike Graglia is the Managing Director & Co-Founder of the SynGAP Research Fund (SRF — https://www.syngapresearchfund.org/), an organization that he set up in 2018 with his wife Ashley, after their son was diagnosed with a rare neurological disease caused by an insufficiency in SynGAP protein, which causes the life-changing diagnoses of Epilepsy, Autism, sleep disorder and intellectual disability.
The mission of SRF is to improve the quality of life of SynGAP1 patients through the research and development of treatments, therapies and support systems.
Previously, Mike worked at the Emerson Collective, New America Foundation, the Bill and Melinda Gates Foundation, BCG, the World Bank/IFC and the US Peace Corps/Namibia. During his time in Africa he created a small charity to fund girls’ education.
Mike graduated from Gonzaga University with a BS in mathematics. He then attended the Johns Hopkins School of Advanced International Studies (SAIS) and Columbia Business School.