Inflammation, thought to be a driver of age-related disease, does not worsen with age in some Indigenous communities.


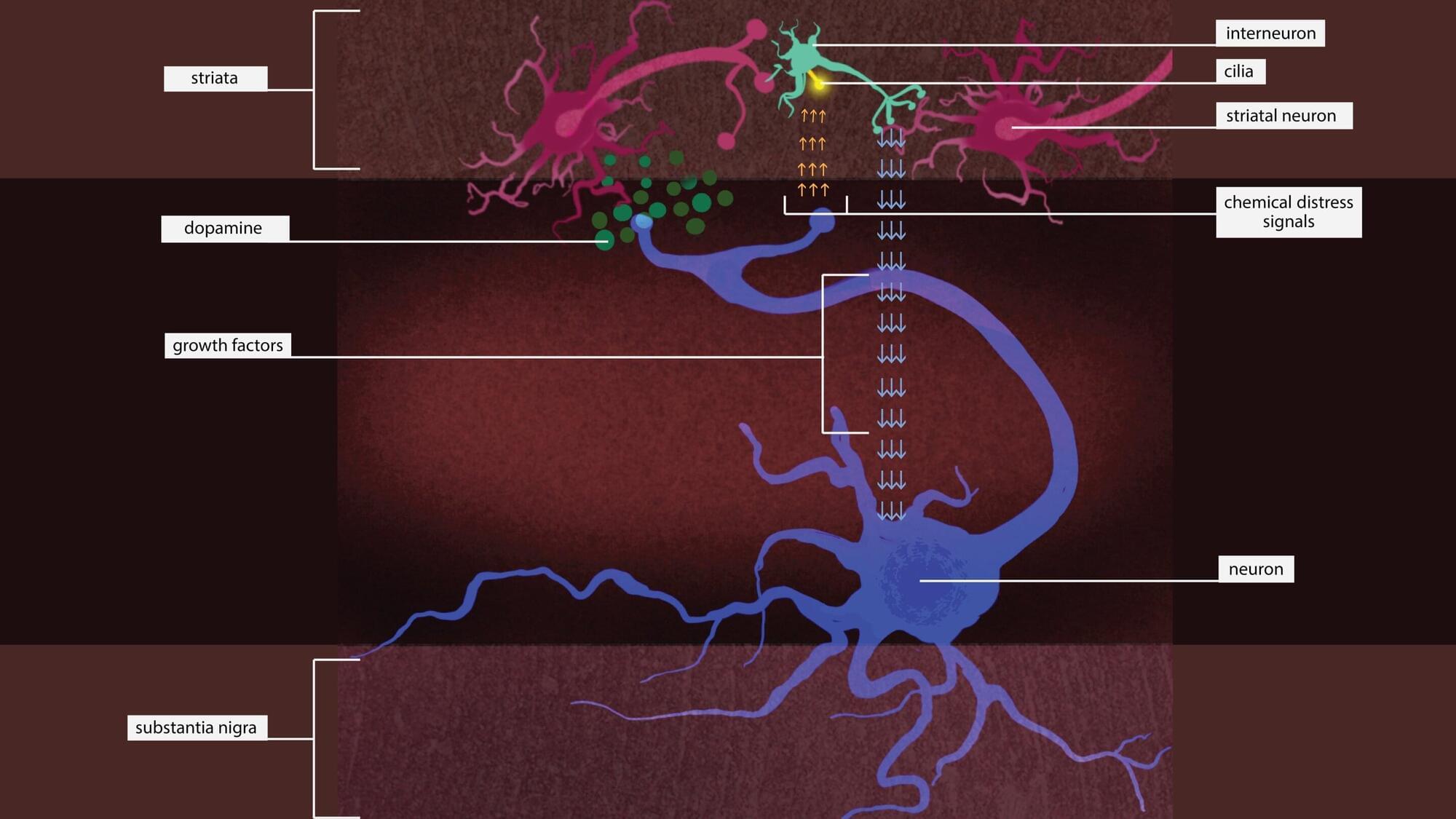
Putting the brakes on an enzyme might rescue neurons that are dying due to a type of Parkinson’s disease that’s caused by a single genetic mutation, according to a new Stanford Medicine-led study conducted in mice.
The study has been published in Science Signaling.
The genetic mutation causes an enzyme called leucine-rich repeat kinase 2, or LRRK2, to be overactive. Too much LRRK2 enzyme activity changes the structure of brain cells in a way that disrupts crucial communication between neurons that make the neurotransmitter dopamine and cells in the striatum, a region deep in the brain that is part of the dopamine system and is involved in movement, motivation and decision-making.
Researchers at UT Arlington have discovered a key enzyme, IDO1, that when blocked, helps immune cells regain their ability to properly process cholesterol—something that breaks down during inflammation. This breakthrough could offer a powerful new way to fight heart disease, diabetes, cancer, and more. By “turning off” this enzyme, the team restored cholesterol absorption in macrophages, potentially stopping disease at the source. Even more promising, they found a second enzyme, NOS, that makes things worse—raising hopes that targeting both could pave the way for transformative treatments for millions suffering from inflammation-driven conditions.

CGT manufacturers need to get better at gathering process data to move manufacturing into the digital age according to a new study [Reptile8488/Getty Images]
Cell and gene therapy (CGT) production will only enter the digital age when the industry gets better at gathering process data, according to researchers, who say closed systems and advanced monitoring technologies will be vital.
Unlike in pharma, the fourth industrial revolution has not reached the CGT sector, according to Aleksander Szarzynski, a researcher at the Vienna University of Technology and co-author of a new study looking at cell and gene therapy production.
To study what happened in the brain during this task, the researchers used functional magnetic resonance imaging, which measures blood flow as a proxy for neural activity. They scanned the brains of 11 participants while they performed the memory task over multiple sessions. By applying a complex decoding model to the imaging data, the researchers were able to estimate not only what participants were remembering but also how uncertain they were about each memory. The model treated neural activity as a probabilistic code, where stronger or more focused patterns of activity reflected more confident memory representations.
The results showed that neural signals in the visual cortex—the area of the brain involved in processing visual information—were more intense for the high-priority memory items. These stronger signals translated to smaller memory errors and greater confidence. On average, participants remembered the high-priority items more accurately and responded more quickly when asked to recall them. Their eye movements were closer to the correct location, and they took less time to respond. These behavioral improvements matched the patterns observed in the brain data.
The study also found that the magnitude of neural activity in the frontal cortex predicted how well participants could distinguish between high-and low-priority memories. This suggests that the frontal cortex plays a regulatory role, sending signals that adjust the strength of memory representations in visual areas depending on how important each item is. In other words, the frontal brain regions help direct the mental spotlight, increasing the “volume” of the memories that matter most.
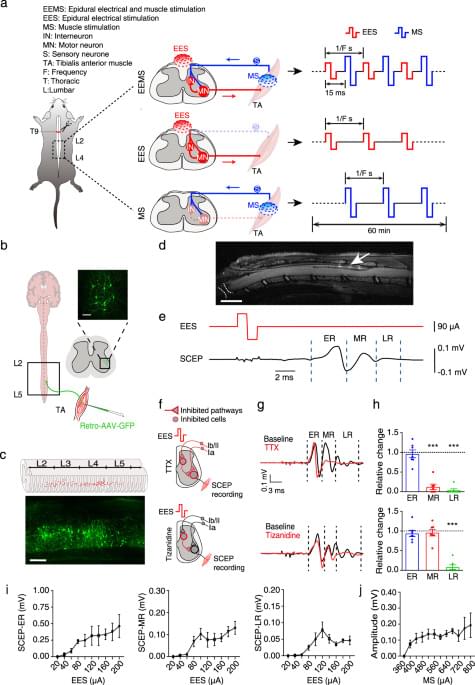
Electrical signals with characteristic parameters for reconstructing neural circuits remain incompletely understood, limiting the therapeutic potential of electrical neuromodulation techniques. Here, the authors demonstrate that dual electrical stimulation at 10–20 Hz rebuilds the spinal sensorimotor neural circuit after spinal cord injury, indicating the characteristic signals of circuit remodeling.
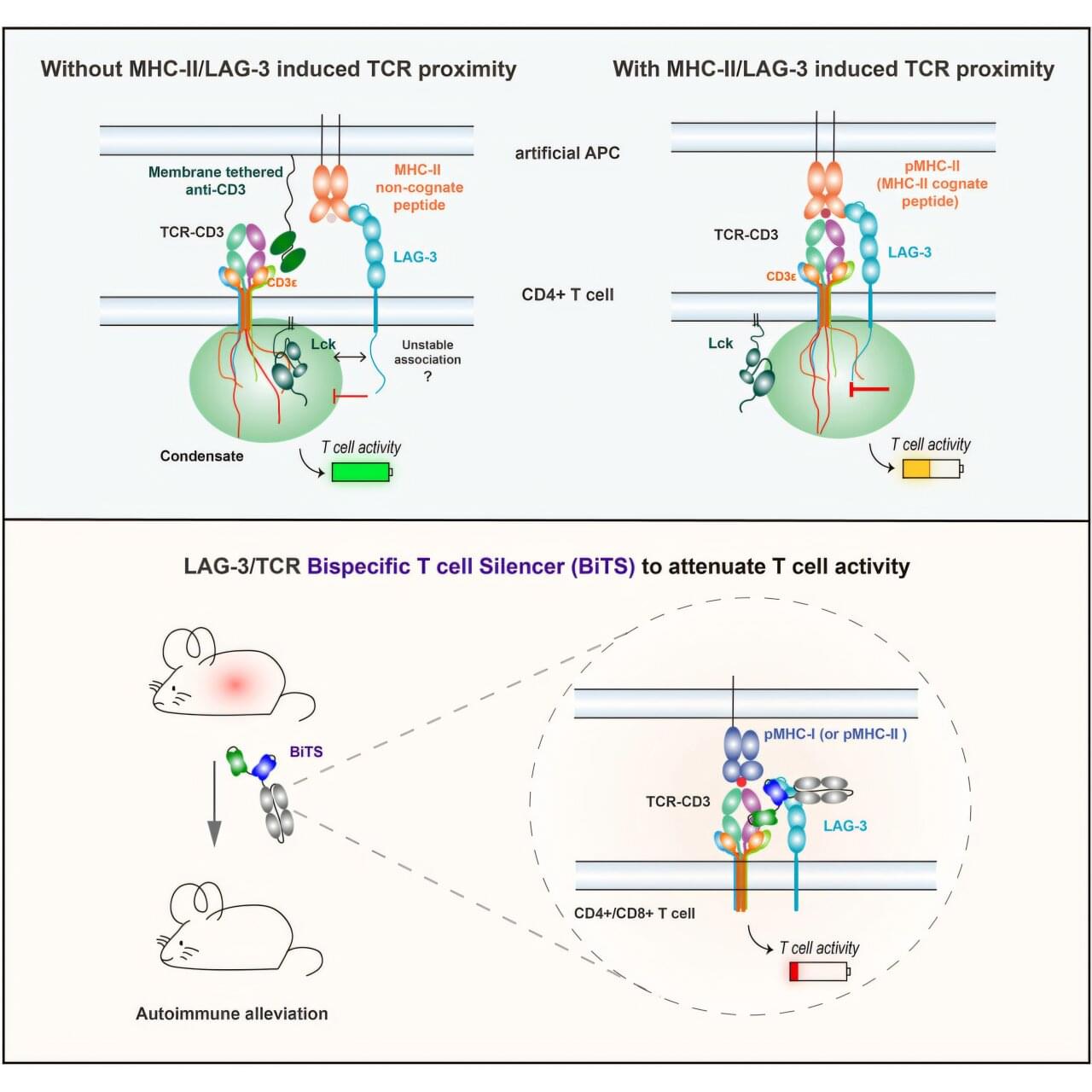
An engineered protein turns off the kind of immune cells most likely to damage tissue as part of type-1 diabetes, hepatitis, multiple sclerosis, shows a new study in mice.
In these autoimmune diseases, T cells mistakenly target the body’s own tissues instead of invading viruses or bacteria as they would during normal immune responses. Treatments focused on T cells have been elusive because blocking their action broadly weakens the immune system and creates risk for infections and cancer.
Published online June 30 in the journal Cell, the study revealed that holding closely together two protein groups (signaling complexes) on T cells, including one found more often on T cells involved in autoimmune disease, shuts down those T cells in a limited way.
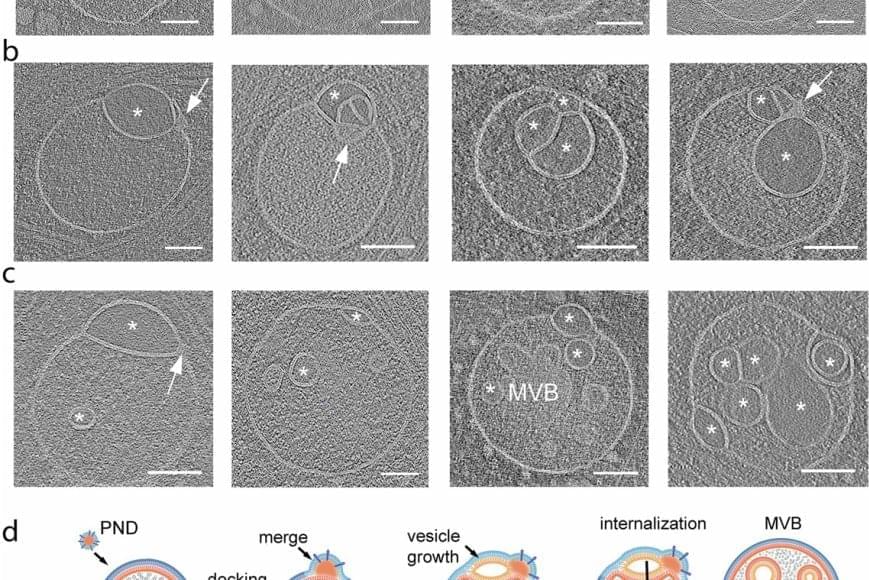
The discovery of an unknown organelle inside our cells could open the door to new treatments for devastating inherited diseases.
The organelle, a type of specialized structure, has been dubbed a “hemifusome” by its discoverers. This little organelle has a big job helping our cells sort, recycle and discard important cargo within themselves, the scientists say. The new discovery could help scientists better understand what goes wrong in genetic conditions that disrupt these essential housekeeping functions.
One such condition is Hermansky-Pudlak syndrome, a rare genetic disorder that can cause albinism, vision problems, lung disease and issues with blood clotting. Problems with how cells handle cargo are at the root of many such disorders.
The scientists believe hemifusomes facilitate the formation of vesicles, tiny blister-like sacs that act as mixing bowls, and of organelles made up of multiple vesicles. This process is critical to cellular sorting, recycling and debris disposal, the researchers report.

Tiny magnetic bots that are activated by light can clear bacterial infections deep in the sinus cavities, then be expelled by blowing out the nose.
A new study published in Science Robotics unveiled copper single–atom–doped bismuth oxoiodide microbots, each smaller than a grain of salt, that can be tracked and guided to the location of infection via X-ray imaging, thus providing a precise, minimally invasive therapeutic strategy for managing sinusitis clinically.
Sinusitis is a common respiratory condition often linked to biofilm produced by bacteria like Streptococcus pyogenes. This condition causes inflammation of the sinus lining and leads to symptoms such as nasal congestion, reduced sense of smell, facial pain, and, in some dire cases, even memory impairment.
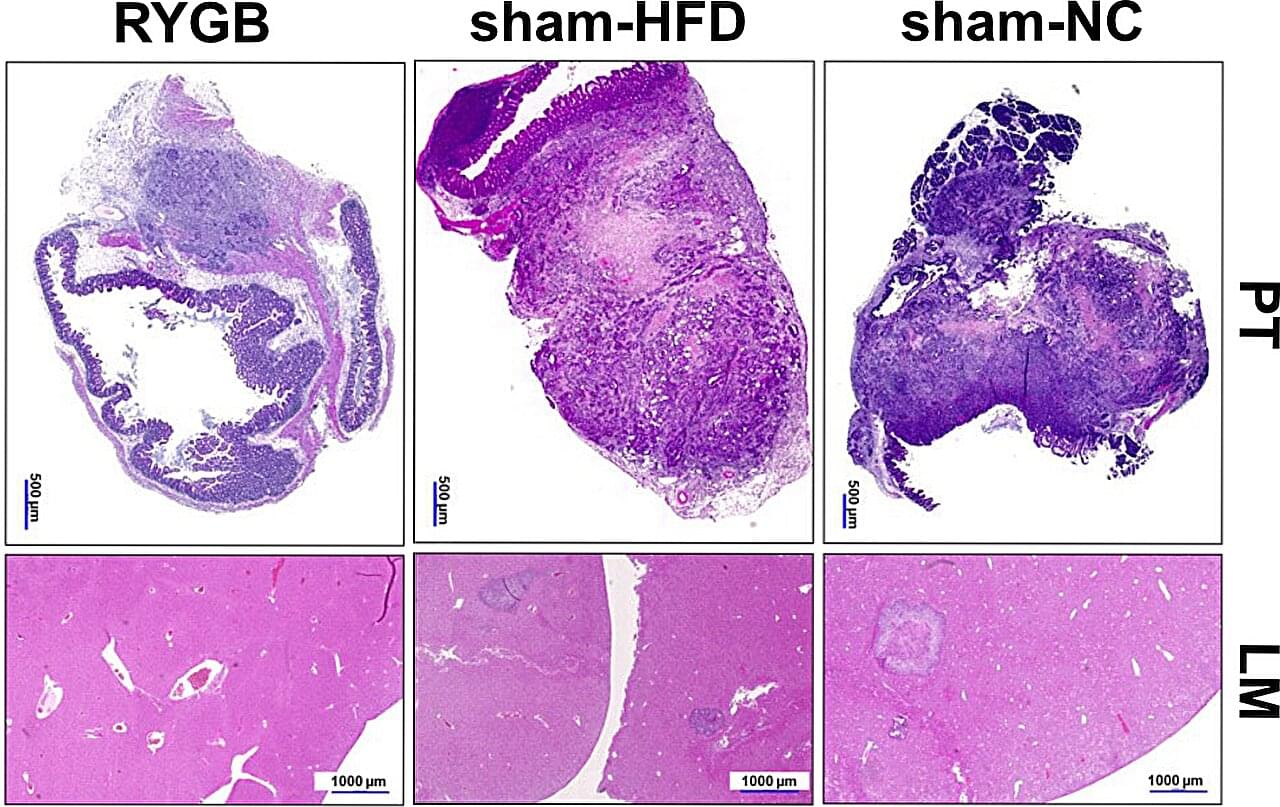
Research led by the Department of General and Visceral Surgery, Faculty of Medicine, University of Freiburg, Germany has found that bile acid diversion in Roux-en-Y gastric bypass (RYGB) reduces colorectal tumor growth and metastasis independent of weight loss, potentially reshaping future cancer treatment approaches.
More than 2 billion adults worldwide are now overweight or obese, a condition marked by chronic low-grade inflammation and metabolic disruption that can promote tumor growth, increasing the risk of developing at least 15 types of cancer. In the United States alone, more than a third of adults face obesity, presenting an urgent public-health crisis.
Among various weight-loss interventions, bariatric surgery, specifically RYGB, is not only effective in promoting sustained weight reduction but has intriguingly been linked to reduced cancer incidence. Whether these changes alone can slow or prevent colorectal cancer remains a question with critical implications for prevention and treatment.