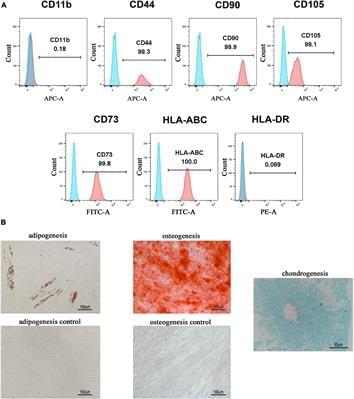
Circa 2021 Secretomes of human pluripotent stem cell-derived smooth muscle cell progenitors upregulate extracellular matrix metabolism in the lower urinary tract and vagina.
Adult mesenchymal stem cells (MSCs) have been studied extensively for regenerative medicine; however, they have limited proliferation in vitro, and the long culture time induces cell senescence. MSCs also contribute to tissue repair through their paracrine function. In this study, we sought to examine the paracrine effects of human smooth muscle cell progenitors (pSMC) on the urethra and adjacent vagina of stress urinary incontinence rodents. We use human pluripotent stem cell (PSC) lines to derive pSMCs to overcome the issue of decreased proliferation in tissue culture and to obtain a homogenous cell population.
Three human PSC lines were differentiated into pSMCs. The conditioned medium (CM) from pSMC culture, which contain pSMC secretomes, was harvested. To examine the effect of the CM on the extracellular matrix of the lower urinary tract, human bladder smooth muscle cells (bSMCs) and vaginal fibroblasts were treated with pSMC-CM in vitro. Stress urinary incontinence (SUI) was induced in rats by surgical injury of the urethra and adjacent vagina. SUI rats were treated with pSMC-CM and monitored for 5 weeks. Urethral pressure testing was performed prior to euthanasia, and tissues were harvested for PCR, Western blot, and histological staining. Kruskal-Wallis one-way ANOVA test and Student t test were used for statistical comparisons.
pSMC-CM upregulated MMP-2, TIMP-2, collagen, and elastin gene expression, and MMP-9 activity in the human bladder and vaginal cells consistent with elastin metabolism modulation. pSMC-CM treatment in the SUI rat improved urethral pressure (increase in leak point pressure compared to intact controls, p 0.05) and increased collagen and elastin expression in the urethra and the adjacent vagina.