An artificial pancreas originally developed at the University of Virginia Center for Diabetes Technology improves blood sugar control in children ages 2 to 6 with type 1 diabetes, according to a new study. Details of the clinical study and its findings have been published in the New England Journal of Medicine.
Trial participants using the artificial pancreas spent approximately three more hours per day in their target blood sugar range compared with participants in a control group who continued relying on the methods they were already using to manage their blood sugar.
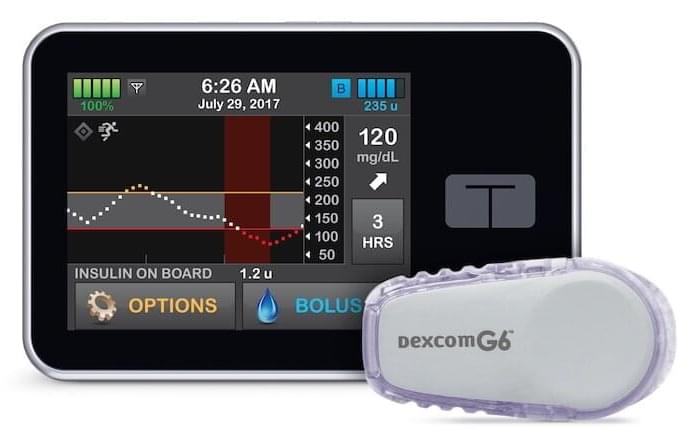
The Control-IQ system, manufactured by Tandem Diabetes Care, is a diabetes management device that automatically monitors and regulates blood glucose. The artificial pancreas has an insulin pump that uses advanced control algorithms based on the person’s glucose monitoring information to adjust the insulin dose as needed.