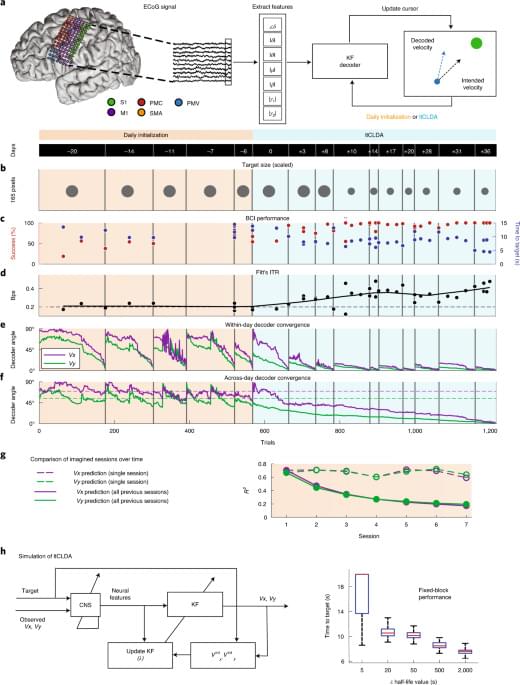
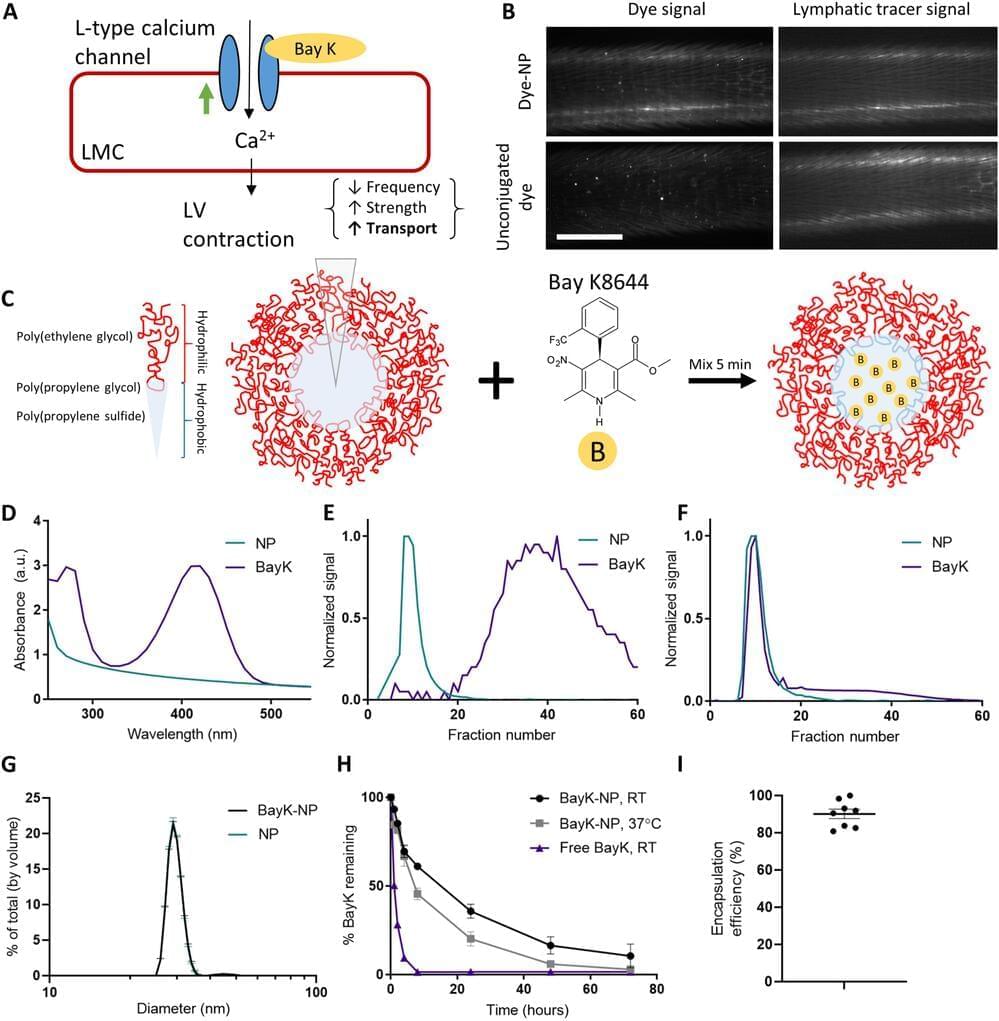
Organoids are an incredible tool for research into the brain. Cerebral organoids are created by growing human stem cells in a bioreactor. They might be the key to unlocking the answers to many of our questions about the brain. We explain how they’re made and some of the discoveries they’ve helped with so far!
–
✍ Script by Duranka Perera (https://www.durankaperera.com/)
✍ Thumb by “Broken” Bran — https://twitter.com/BranGSmith.
–
Support us on Patreon: https://www.patreon.com/Brainbook_
Thank you to our supporters:
Morag Forbes.
Patrick Kohl.
Ronald Coleman Dees.
Alex Rofini.
Helen Whitley.
–
Discover more on our website.
https://www.brainbookcharity.org.
–
Follow us on:
Twitter: https://www.twitter.com/realbrainbook.
Instagram: https://www.instagram.com/brainbook_
Facebook: https://www.facebook.com/realbrainbook