Electron tunneling associated with ferritin was proposed as early as 1988, but it is still viewed skeptically despite substantial evidence that it occurs. In our recent paper published in IEEE Transactions on Molecular, Biological and Multi-Scale Communications, my co-authors and I review the evidence of electron tunneling in ferritin, as well as the evidence that such electron tunneling may be used by biological systems that include the retina, the cochlea, macrophages, glial cells, mitochondria and magnetosensory systems.
While these diverse systems fall in different fields of study, we hope that this article will raise awareness of the mechanism of electron tunneling associated with ferritin and encourage further research into that phenomenon in biological systems that incorporate ferritin, particularly where there is no apparent need for the iron storage functions of ferritin in those systems.
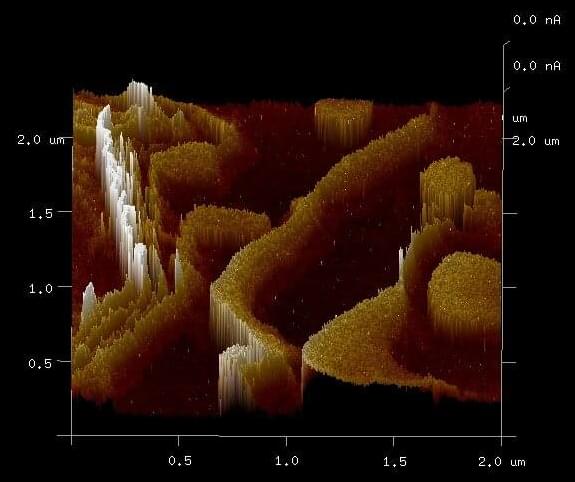
Ferritin is an iron storage protein that self-assembles into a 12-nanometer diameter spherical shell that is 2 nanometers thick, and it can store up to ~4,500 iron atoms in an 8-nanometer diameter core. With an evolutionary history that appears to stretch back more than 1.2 billion years, it might seem rather old, but it should be kept in mind that single-celled organisms are believed to have first evolved ~3.5 billion years ago. As such, it may have taken more than 2 billion years for ferritin to evolve. When the first multicellular organisms evolved ~600 million years ago, members of the ferritin family of proteins were likely present, and they can be found today in almost all plants and animals.