And he mentions Internatioan Aging Systems where you can legally order Rapamycin but I would have to know what experience people have had with them and their product first myself.
Magnesium Breakthrough 10% Discount _https://bit.ly/3O5tPfu_ Code Modern10 This video brought to you by BiOptimizers.
Some links are affiliate links so we will earn a commission when they are used to purchase products.
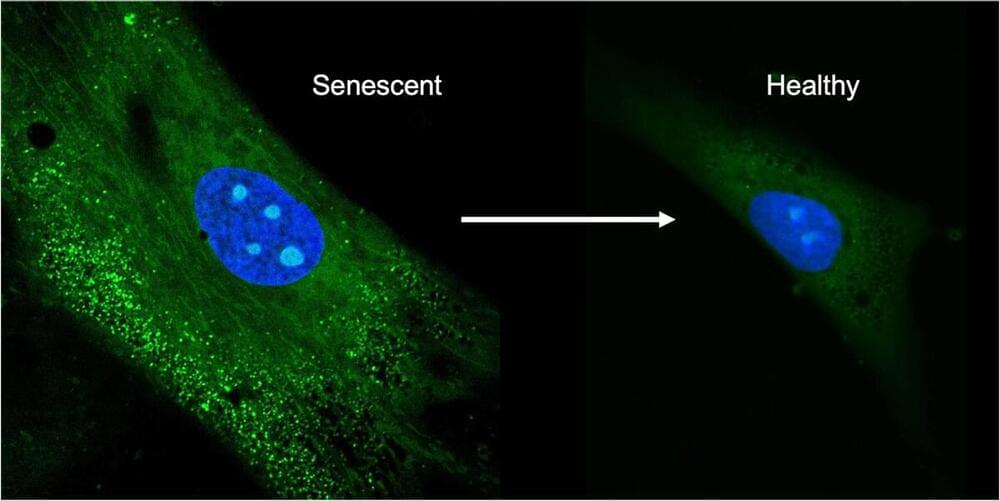
In this video Pelton talks all about rapamycin, its history, how it works, dosage and timing. He also talks about where you can get rapamycin online.
Ross Pelton is a pharmacist, nutritionist, author, and health educator. He is the founder of The Natural Pharmacist, a website and online resource that provides information about natural health and nutrition. Pelton is also the author of several books, including The Drug-Induced Nutrient Depletion Handbook, The Nutritional Cost of Drugs, and Alternatives in Cancer Therapy. He is a recognized expert on drug-induced nutrient depletions and has been featured in publications such as The New York Times, The Wall Street Journal, and Time magazine.
00:00 History of rapamycin.
03:00 Rapamycin for life extension.
05:50 mTOR & autophagy.
14:30 mTOR research.
16:00 Who can & can’t take rapamycin.
22:40 Rapamycin research & info.
24:50 Metrics to watch.
26:55 How to take rapamycin.
29:09 Getting started with rapamycin.
Dr Ross Pelton’s links.