Several factors contribute to the development of inflamm-aging, including genetic susceptibility, visceral obesity, microbiota and gut permeability, cellular senescence, NLRP3 inflammasome activation, oxidative stress caused by mitochondrial dysfunction, immune cells dysregulation, and chronic infection (Ferrucci & Fabbri, 2018). The immune system becomes gradually dysregulated during aging, leading to elevated blood levels of pro-inflammatory mediators, such as TNFα, IL6, and C-reactive protein (Harris et al., 1999 ; Mooradian et al., 1991). Energy homeostasis also becomes dysregulated with aging, which results in the redistribution of subcutaneous fat to visceral regions and contributes to inflammation (Bouchard et al., 1993 ; Chumlea et al., 1989 ; Curtis et al., 2005). Metabolism-induced inflammation, also known as metaflammation, shares similarities with inflamm-aging, including the elevation of certain circulating pro-inflammatory cytokines (Prattichizzo et al., 2018). Therefore, the molecules that play a key role in the regulation of metabolic homeostasis potentially mediate the development of chronic inflammation during aging.
Forkhead box O1 (FOXO1) transcription factor has been indicated to be involved in the regulation of nutrient metabolism and energy homeostasis (Cheng et al., 2009 ; InSug et al., 2015 ; Matsumoto et al., 2007 ; Yang et al., 2019 ; Zhang et al., 2012). Deletion of hepatic Foxo1 improves glucose homeostasis in insulin resistant mice (Dong et al., 2008). FOXO1 inhibition by AS1842856 attenuates hepatic steatosis in diet-induced obesity mice (Ding et al., 2020). In mature macrophages, FOXO1 promotes inflammation through the activation of TLR4-and STAT6-mediated signaling pathways (Fan et al., 2010 ; Lee et al., 2022). In invertebrates, DAF-16, the Foxo homolog gene, mediates the effect of insulin/IGF signaling on lifespan (Ogg et al., 1997). Overexpression of FOXO in Drosophila and C.elegans increases their lifespan (Giannakou et al., 2004 ; Henderson & Johnson, 2001). However, studies in mammalians show that FOXO1 does not have a significant correlation with longevity (Chiba et al., 2009 ; Kleindorp et al., 2011). Considering the role of FOXO1 in regulating glucose metabolism and inflammation, we hypothesize that FOXO1 plays an important role in the regulation of aging-induced inflammation and dysregulation of glucose homeostasis.
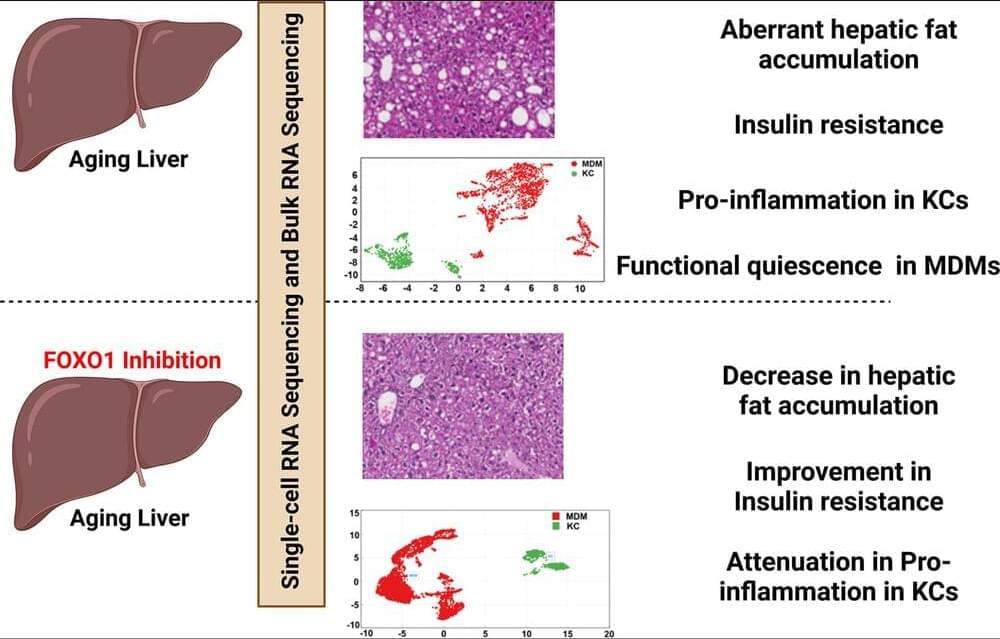
Liver is an important metabolic organ that plays a key role in maintaining whole-body nutrient homeostasis by regulating energy metabolism, clearing xenobiotic and endobiotic, and synthesizing necessary molecules (Rui, 2014). As a result, aging-induced changes in liver contribute to systemic susceptibility to aging-related diseases. Different types of liver cells, including hepatocytes, endothelial cells, hepatic stellate cells (HSC), and macrophages, are all affected by the aging process (Hunt et al., 2019). However, most studies on liver aging focused on whole-liver tissue, which is mainly composed of parenchymal cells, hepatocytes. Thus, the effects of aging on liver nonparenchymal cells (NPCs) are less understood. In this study, we used bulk RNA-Seq and single-cell RNA (scRNA)-Seq technologies to analyze aging-induced changes, and the role of FOXO1 in aging-related processes in both whole-liver and individual liver cells, particularly liver macrophages. We found that insulin resistance, liver fat accumulation, liver inflammation, and systemic inflammation were significantly aggravated in old mice. Additionally, aging significantly increased pro-inflammatory response in Kupffer cells (KCs) and induced a functional quiescence in monocyte-derived macrophages (MDMs). FOXO1 activity was significantly enhanced in the livers of old mice and FOXO1 inhibition improved insulin resistance, hepatic steatosis, and inflammation in old mice. Furthermore, we found that FOXO1 inhibition attenuated aging-induced pro-inflammation in KCs and had a limited effect on aging-induced functional quiescence in MDMs. Taken together, this study indicates that FOXO1 plays an important role in the liver aging processes and suggests that FOXO1 is a potential therapeutic target for the treatment of aging-induced chronic diseases.