The Food and Drug Administration May 16 announced it cleared the first blood test to diagnose Alzheimer’s disease.



Antler blastema progenitor cells (ABPCs) are a distinct population of skeletal mesenchymal stem cells found in regenerating deer antlers, with strong stemness and renewal capacity in vitro. Stem cell-derived extracellular vesicles (EVs) are emerging as potential therapeutic candidates that can mediate donor cells’ beneficial effects. Here, we tested the effects of ABPC-derived EVs (EVsABPC) on aging in mice and rhesus macaques (Macaca mulatta). We identified a variety of unique factors in EVsABPC and showed that in vitro, EVsABPC attenuated phenotypes of senescence in bone marrow stem cells. In aged mice and macaques, EVsABPC substantially increased femoral bone mineral density. Further, intravenous EVsABPC improved physical performance, enhanced cognitive function and reduced systemic inflammation in aged mice, while reversing epigenetic age by over 3 months. In macaques, EVABPC treatment was also neuroprotective, reduced inflammation, improved locomotor function and reduced epigenetic age by over 2 years. Our findings position ABPCs as an emerging and practical source of EVs with translational value for healthy aging interventions.
Inspired by the regenerative capacity of deer antlers, Hao and colleagues report that antler blastema progenitor cell-derived extracellular vesicle treatment counteracts bone loss and epigenetic aging and is neuroprotective in mice and macaques.
Type I diabetes is an exhausting full-time job. Having it means living a life full of constant care and maintenance. You’re always checking in on your blood sugar to make sure it isn’t too high or is it too low, extremes that could be easily reached with the most minor indulgence or tiniest bout of laziness.
A new treatment, as detailed in the New York Times, might change everything we know about managing type I diabetes.
Zimislecel is an experimental stem cell-based therapy that recently dropped a bomb on the diabetes world. Developed by the Boston-based Vertex Pharmaceuticals, it’s a one-time infusion that has turned 10 of 12 trial patients suffering from severe type I diabetes into people who no longer need insulin, and in less than a year.

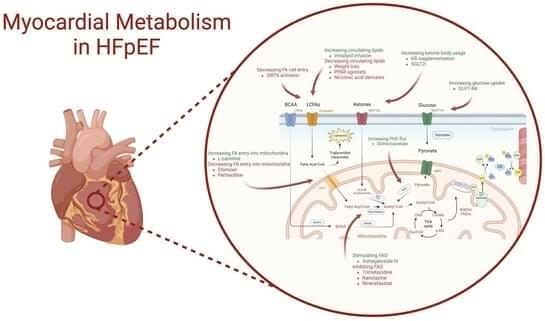
Heart failure with preserved ejection fraction (HFpEF) is increasingly prevalent and now accounts for half of all heart failure cases. This rise is largely attributed to growing rates of obesity, hypertension, and diabetes. Despite its prevalence, the pathophysiological mechanisms of HFpEF are not fully understood. The heart, being the most energy-demanding organ, appears to have a compromised bioenergetic capacity in heart failure, affecting all phenotypes and aetiologies. While metabolic disturbances in heart failure with reduced ejection fraction (HFrEF) have been extensively studied, similar insights into HFpEF are limited. This review collates evidence from both animal and human studies, highlighting metabolic dysregulations associated with HFpEF and its risk factors, such as obesity, hypertension, and diabetes.
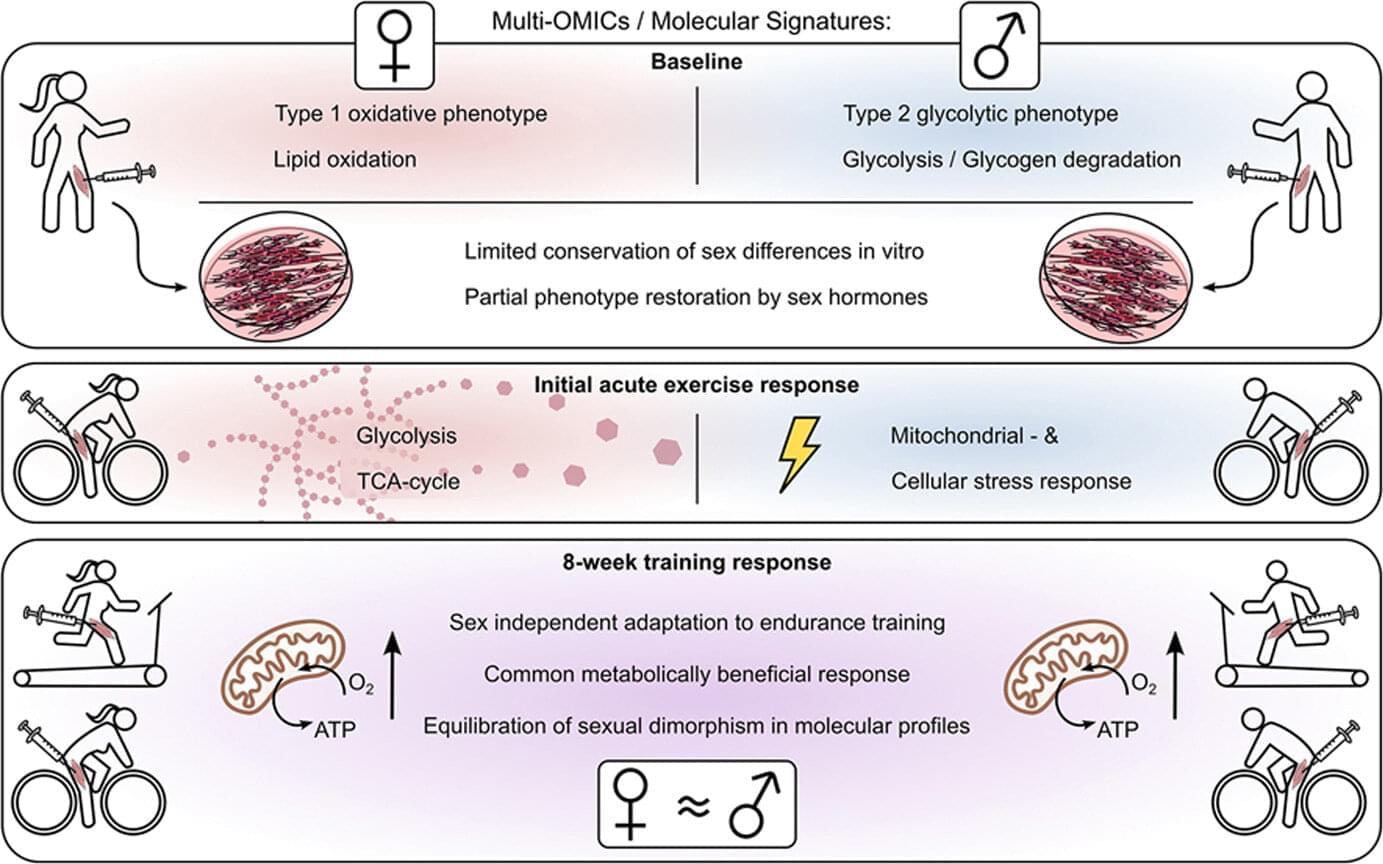
The skeletal muscles of men and women process glucose and fats in different ways. A study conducted by the University Hospital of Tübingen, the Institute for Diabetes Research and Metabolic Diseases of Helmholtz Munich and the German Center for Diabetes Research (DZD) e. V. provides the first comprehensive molecular analysis of these differences. The results, published in Molecular Metabolism, possibly give an explanation for why metabolic diseases such as diabetes manifest differently in women and men—and why they respond differently to physical activity.
Skeletal muscles are far more than just “movement driving motors.” They play a central role in glucose metabolism and therefore also in the development of type 2 diabetes. This is due to the fact that around 85% of insulin-dependent glucose uptake takes place in the muscles.
This means that if muscle cells react less sensitively to insulin, for example in the case of insulin resistance, glucose is less easily absorbed from the blood. This process is specifically counteracted by physical activity.
In a major advance for patients with Crohn’s disease, a new study led by researchers at Mount Sinai Health System found that guselkumab, a medication with a mechanism of action that is new to inflammatory bowel disease (IBD) treatment, outperformed an established standard of care in promoting intestinal healing and symptom relief.
These findings from two pivotal Phase III trials known as GALAXI 2 and 3, published in The Lancet, provided the basis for the recent Food and Drug Administration approval of guselkumab (brand name Tremfya) for the treatment of moderately to severely active Crohn’s disease.
Crohn’s disease affects roughly 780,000 people in the United States and often requires a lifetime of management. Despite numerous available biologic medications, many patients fail to achieve sustained remission. Guselkumab blocks the interleukin-23 (IL-23) pathway, a key driver of chronic intestinal inflammation.

Teeth may seem like static fixtures, but a new collaboration between engineers and clinicians is proving just how dynamic, informative and medically significant our teeth can be.
In a study, published in ACS Applied Materials & Interfaces, engineers and dentists come together to uncover how teeth, as biological material, hold key information for understanding rare craniofacial disorders that develop during childhood.
Kyle Vining, Assistant Professor in Materials Science and Engineering (MSE) and in Preventive and Restorative Science at Penn Dental Medicine, leads this interdisciplinary team, which includes Yuchen (Tracy) Jiang, a former master’s student in MSE, Kei Katsura, a pediatric dentist and KL2 postdoctoral research scholar at Children’s Hospital of Philadelphia (CHOP) and the Institute of Translational Medicine and Therapeutics at Penn, and Elizabeth Bhoj, Assistant Professor of Pediatrics in Penn Medicine and the Division of Human Genetics at CHOP.

Vitamin D is not only an essential nutrient, but also the precursor of the hormone calcitriol, indispensable for health. It regulates the uptake of phosphate and calcium necessary for bones by the intestines, as well as cell growth and the proper function of muscles, nerve cells, and the immune system.
Now, researchers have shown for the first time in Frontiers in Endocrinology that a particular gene called SDR42E1 is crucial for taking up vitamin D from the gut and further metabolizing it—a discovery with many possible applications in precision medicine, including cancer therapy.
“Here we show that blocking or inhibiting SDR42E1 may selectively stop the growth of cancer cells,” said Dr. Georges Nemer, a professor and associate dean for research at the University College of Health and Life Sciences at Hamad Bin Khalifa University in Qatar, and the study’s corresponding author.