Google DeepMind has used its technology to identify parts of human DNA that might cause diseases.


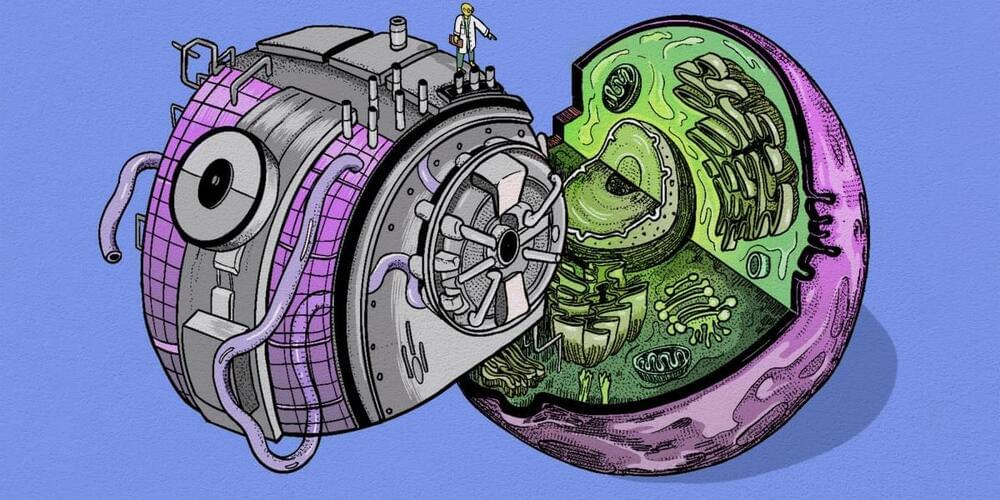
A virtual cell modeling system, powered by AI, will lead to breakthroughs in our understanding of diseases, argue the cofounders of the Chan Zuckerberg Initiative.
As the smallest living units, cells are key to understanding disease—and yet so much about them remains unknown. We do not know, for example, how billions of biomolecules—like DNA, proteins, and lipids—come together to act as one cell. Nor do we know how our many types of cells interact within our bodies. We have limited understanding of how cells, tissues, and organs become diseased and what it takes for them to be healthy.
AI can help us answer these questions and apply that knowledge to improve health and well-being worldwide—if… More.
CHOP researchers established the feasibility of an artificial womb called the “Biobag” to nurture a premature lamb in 2017.
The US Food and Drug Administration (FDA) will hold a meeting of independent advisors on September 19–20. The meeting’s agenda is to discuss the viability of clinical trials using artificial womb technology to improve the survival and health of extremely preterm newborns.
Reportedly, during this meeting, regulators and experts will delve into ethical concerns and evaluate various crucial aspects, including the potential steps and design of human trials for this technology.

Get my FREE guide 3 Steps to Reverse Aging when you sign up for my weekly health picks 👉 https://bit.ly/IncreaseHealthspan.
There is powerful science behind how our beliefs inform our genetic expression. It’s not our genes alone that dictate our health outcomes, rather it’s the biology of belief that determines our destiny.
Today on The Doctor’s Farmacy, I’m excited to talk to Dr. Bruce Lipton about how exactly our thoughts determine our genetic expression, and how we can influence our health using our minds.
Dr. Bruce Lipton is a stem cell biologist and author of the bestselling books, The Biology of Belief, Spontaneous Evolution, and The Honeymoon Effect. Dr. Lipton is the recipient of the prestigious Japanese Goi Peace Award and has been listed in the top 100 of “the world’s most spiritually influential people” by Briton’s Watkins Journal for the last 13 years.
This episode is brought to you by Rupa Health, BiOptimizers, LMNT, and Apollo.
Rupa Health is a place where Functional Medicine practitioners can access more than 3,000 specialty lab tests from over 35 labs like DUTCH, Vibrant America, Genova, and Great Plains. You can check out a free, live demo with a Q&A or create an account at https://RupaHealth.com.
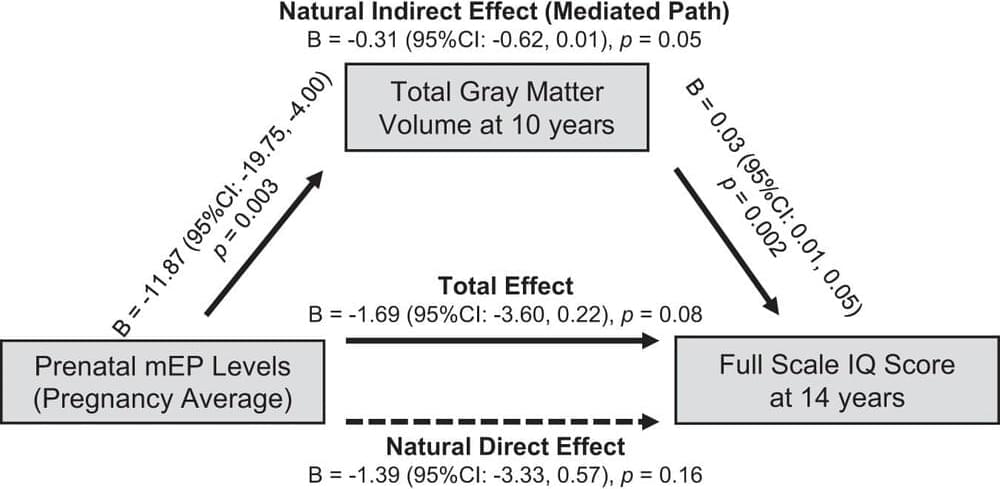
Children whose mothers had a higher exposure to certain phthalates during pregnancy tend to show smaller total gray matter in their brains at age 10. This is one of the main conclusions of a study led by the NYU Grossman School of Medicine and the Barcelona Institute for Global Health (ISGlobal), and published in Molecular Psychiatry.
The study also found that maternal exposure to plasticizers during pregnancy is associated with lower child IQ at age 14, which confirmed the results of two previous study on the topic. Moreover, the research team observed that this relationship between exposure to certain phthalates and lower child IQ is partially influenced by total gray matter volumes. In other words: exposure to plasticizers before birth could lead to smaller total gray matter in childhood, which in turn could be related to a lower IQ.
Finally, the results showed an association between gestational exposure to plasticizers and smaller white matter volumes in girls.

Traumatic brain injury (TBI) is a leading cause of long-term disability and premature death, especially among military personnel and those playing contact sports. Substantial research has examined acute and chronic neurological consequences of TBI; however, non-neurological conditions associated with TBI are understudied.
A new review paper by investigators from Mass General Brigham presents key findings on long-term associations between TBI and cardiovascular disease, highlighting that nervous system dysfunction, neuroinflammation, changes in the brain-gut connection, and post-injury comorbidities may elevate risk of both cardiovascular and cognitive dysfunction in TBI survivors compared to the general population.
The review, published in The Lancet Neurology, emphasizes the need for future cardiovascular research, surveillance and intervention in TBI survivors.
Psychoses like schizophrenia cost billions of dollars annually and derail the lives of people struggling with the disease. Now Monash University researchers have modeled how the effects of psychosis spread through the brain, allowing them to isolate areas where these changes may originate from and which could be targeted by therapies designed to reduce the disease’s progression.
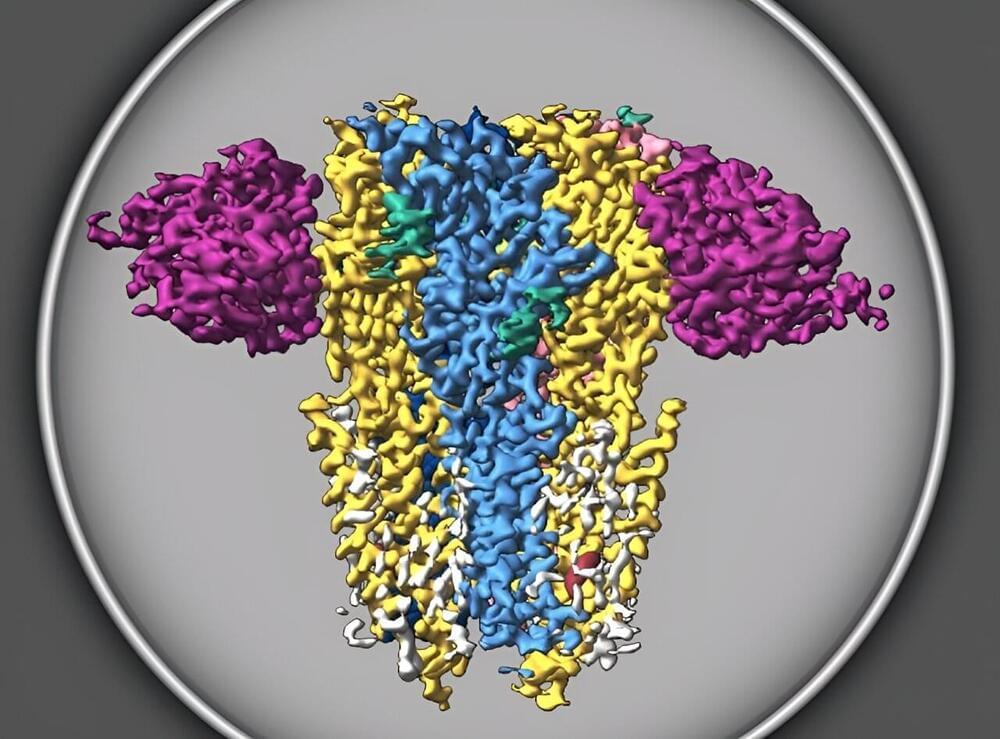
Scientists have revealed the molecular structure of a type of receptor that’s crucial to brain development and function.
Known as Type A GABA receptors, these receptors are already targeted by pharmaceutical anesthetics, sedatives and antidepressants because of their important role in brain function. The discovery, published today in the journal Nature, reveals the dominant assemblies and states of the GABA receptor, a finding that could enable the development of new compounds that more specifically target a range of medical disorders.
“It is the main player that balances excitation and inhibition in the brain,” said lead author Chang Sun, Ph.D., a postdoctoral researcher in the Vollum Institute at Oregon Health & Science University. “It affects all aspects of brain function, from motor function, to memory and learning, and also emotion and anxiety.”