Cell Press
In a recent study published in Cancer Cell, researchers combined deep microbial sequencing and targeted culturing of bacteria with in vitro assessments to investigate tumor and gut microbiome traits that impact chemoradiation therapy in patients with cervical cancer.
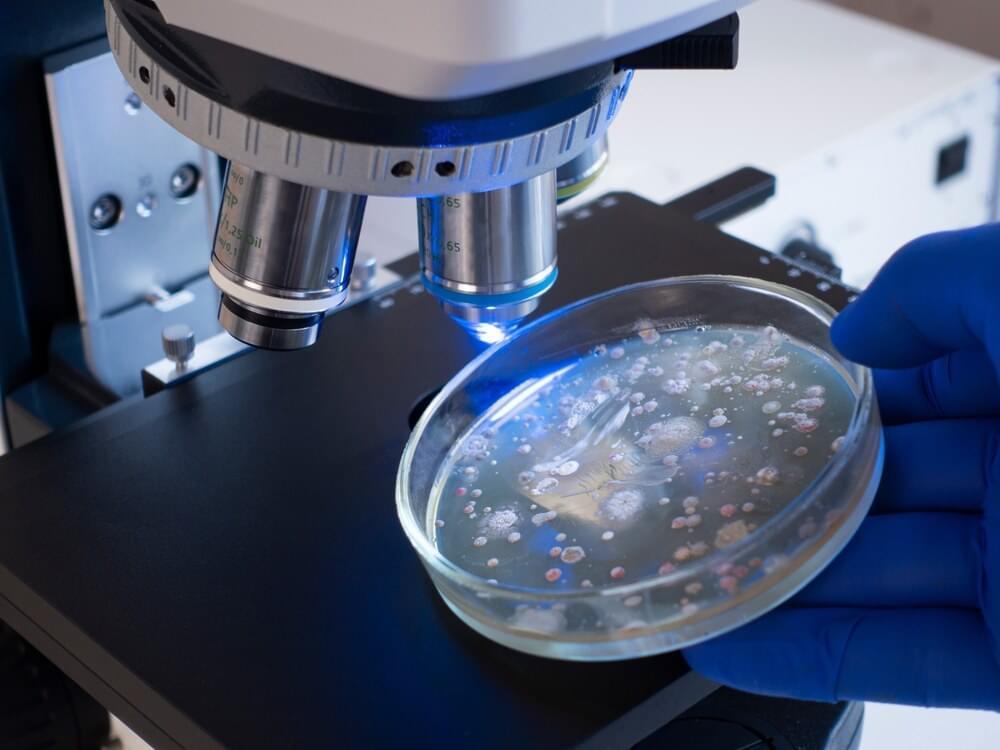
Study: Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Image Credit: Prrrettty/Shutterstock.com.
Background
Cancer and immune cell signaling, metabolism, and proliferation can all be affected by active metabolites produced by tumor microbiota. Tumors, especially in germ-free organs, have distinct microbiomes influencing response to therapy and patient survival.