Biologists have just succeeded in restoring some biological functions in a cell belonging to awoolly mammoth that lived 28,000 years ago.

Biologists have just succeeded in restoring some biological functions in a cell belonging to awoolly mammoth that lived 28,000 years ago.


NEW ORLEANS—Exposure to the widely used chemical bisphenol A (BPA) during pregnancy, even at levels lower than the regulated “safe” human exposure level, can lead to changes in circadian rhythms, according to a mice study to be presented Monday at ENDO 2019, the Endocrine Society’s annual meeting in New Orleans, La. The researchers report these changes may be a contributing factor in hyperactivity seen in BPA-exposed mice.
“The hypothalamus, which we have identified as a brain region that is particularly susceptible to developmental disruption by BPA, contains the site of the clock cells that govern daily rhythms throughout the body,” said researcher Deborah Kurrasch, Ph.D., Associate Professor at the University of Calgary in Calgary, Canada. “We have shown in previous research that BPA exposure in utero can cause defects to the development of hypothalamic nuclei and hyperactivity, and here we explored whether a shift in circadian biology might explain why the animals moved more.”
BPA is a chemical that is added to many commercial products, including water bottles, paper receipts, can liners and food storage containers. It is known as an endocrine-disrupting chemical—a chemical that interferes with the body’s hormones.
New research from Washington University in St. Louis explains the cellular processes that allow a sun-loving microbe to “eat” electricity—transferring electrons to fix carbon dioxide to fuel its growth.
Led by Arpita Bose, assistant professor of biology in Arts & Sciences, and Michael Guzman, a Ph.D. candidate in her laboratory, a Washington University team showed how a naturally occurring strain of Rhodopseudomonas palustris takes up electrons from conductive substances like metal oxides or rust. The work is described in a March 22 paper in the journal Nature Communications.
The study builds on Bose’s previous discovery that R. palustris TIE-1 can consume electrons from rust proxies like poised electrodes, a process called extracellular electron uptake. R. palustris is phototrophic, which means that it uses energy from light to carry out certain metabolic processes. The new research explains the cellular sinks where this microbe dumps the electrons it eats from electricity.

Another step towards organic ships?
Inspired by jellyfish, researchers have created an electronic skin that is transparent, stretchable, touch-sensitive, and repairs itself in both wet and dry conditions. The novel material has wide-ranging uses, from water-resistant touch screens to soft robots aimed at mimicking biological tissues.

Cells from a woolly mammoth that died 28,000 years ago have begun to show “signs of biological [activity]” after they were implanted in mouse cells. However, researchers caution that it’s unlikely the extinct creatures will walk the Earth again anytime soon.
The research, published in Scientific Reports, details how a well-preserved woolly mammoth, found in 2011 in the Siberian permafrost, has begun to show some activity.

Scientists at the Marine Biological Laboratory (MBL) have identified gene “partners” in the axolotl salamander that, when activated, allow the neural tube and associated nerve fibers to functionally regenerate after severe spinal cord damage. Interestingly, these genes are also present in humans, though they are activated in a different manner. Their results are published this week in Nature Communications Biology.

Well, Wesley J Smith just did another hit piece against Transhumanism. https://www.nationalreview.com/corner/transhumanism-the-lazy…provement/
It’s full of his usual horrible attempts to justify his intelligent design roots while trying to tell people he doesn’t have any religious reasons for it. But, then again, what can you expect from something from the National Review.
Sometimes you have to laugh. In “Transhumanism and the Death of Human Exceptionalism,” published in Aero, Peter Clarke quotes criticism I leveled against transhumanism from a piece I wrote entitled, “The Transhumanist Bill of Wrongs” From my piece:
Transhumanism would shatter human exceptionalism. The moral philosophy of the West holds that each human being is possessed of natural rights that adhere solely and merely because we are human. But transhumanists yearn to remake humanity in their own image—including as cyborgs, group personalities residing in the Internet Cloud, or AI-controlled machines.
That requires denigrating natural man as exceptional to justify our substantial deconstruction and redesign. Thus, rather than view human beings as exclusive rights-bearers, the [Transhumanist Bill of Rights] would grant rights to all “sentient entities,” a category that includes both the biological and mechanical.
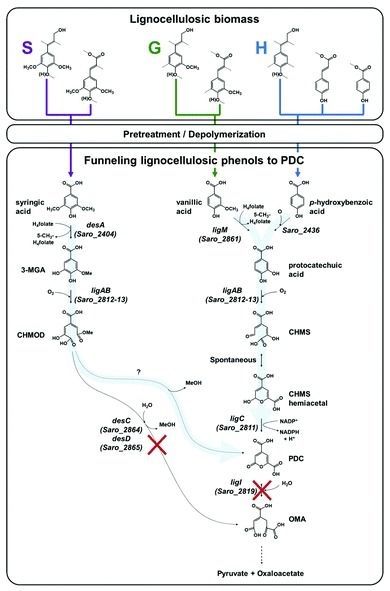
Learning to deal with lignin is important for recycling and space settlements. Unused biomass on space settlements and long-term voyages is something that just can’t be tolerated. The same problem exists in dealing with plant waste on earth. A new process helps convert it into a precursor for polyester, which can be used for all kinds of other materials.
Plant cells are composed of three main substances: cellulose, hemicellulose, and lignin. According to Yining Zeng, Michael E. Himmel, and Shi-You Ding in Biotechnology for Biofuels, the composition amounts to “40 to 50% of cellulose, 15 to 25% hemicelluloses, 20 to 25% lignin, and 5 to 10% other components.[1]” For the most part, the only truly useful part is the cellulose and the hemicellulose. The lignin is usually just thrown away. The most common use is fuel for heating units. That’s right. They just burn it.
We can’t keep doing it that way. However, there really isn’t an alternative. Until now. A recent article in Science Daily referenced a new journal article about the use of Novosphingobium aromaticivorans. This is “genus of Gram-negative bacteria that includes N. taihuense, which can degrade aromatic compounds such as phenol, aniline, nitrobenzene and phenanthrene.[2]” Using genetic engineering, they deleted certain genes which allowed the microbe to convert lignin into 2-pyrone-4–6-dicarboxylic acid, which can be converted into polyester. The detailed information is available for free download and was published under the title “Funneling aromatic products of chemically depolymerized lignin into 2-pyrone-4–6-dicarboxylic acid with Novosphingobium aromaticivorans.[3]”