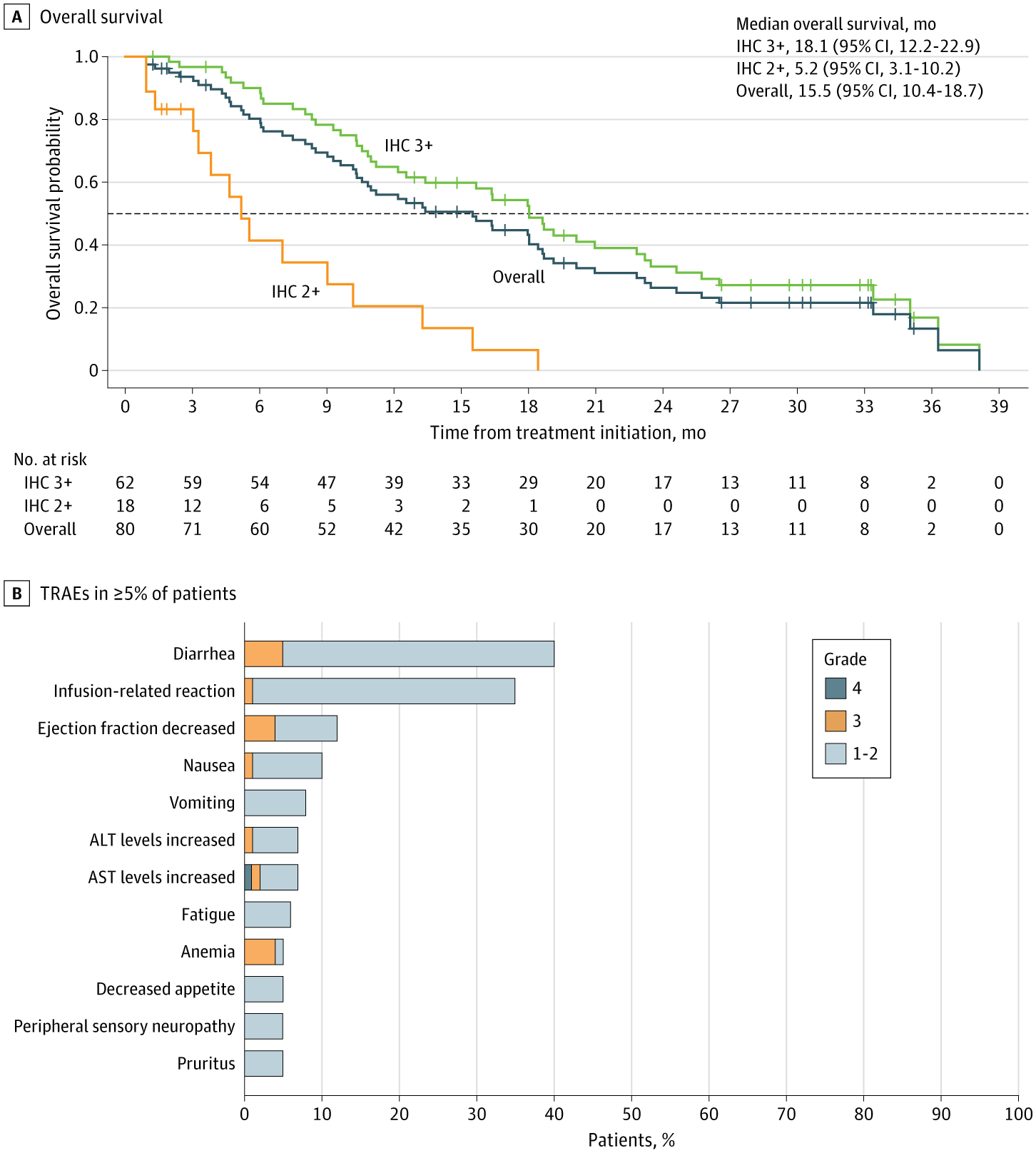
Among adults with treatment-refractory, HER2-positive BiliaryTractCancer, zanidatamab produced sustained, meaningful clinical responses and extended survival compared to prior standards.
In patients with immunohistochemistry (IHC) 3+ tumors, response rates and overall survival were notably higher than those with IHC 2+ tumors, substantiating the use of reflex IHC testing to identify candidates for HER2-targeted therapy.
Safety remained consistent over 33 months of follow-up, and the ongoing HERIZON-BTC-302 phase 3 trial is assessing zanidatamab alongside first-line standard care in this setting.
This follow-up analysis of the phase 2 HERIZON-BTC-01 trial evaluates the efficacy, patient-reported outcomes, and safety profile of zanidatamab in patients with ERBB2-amplified biliary tract cancer with a HER2 immunohistochemistry score of 3+ or 2+ after 33 months of follow-up.