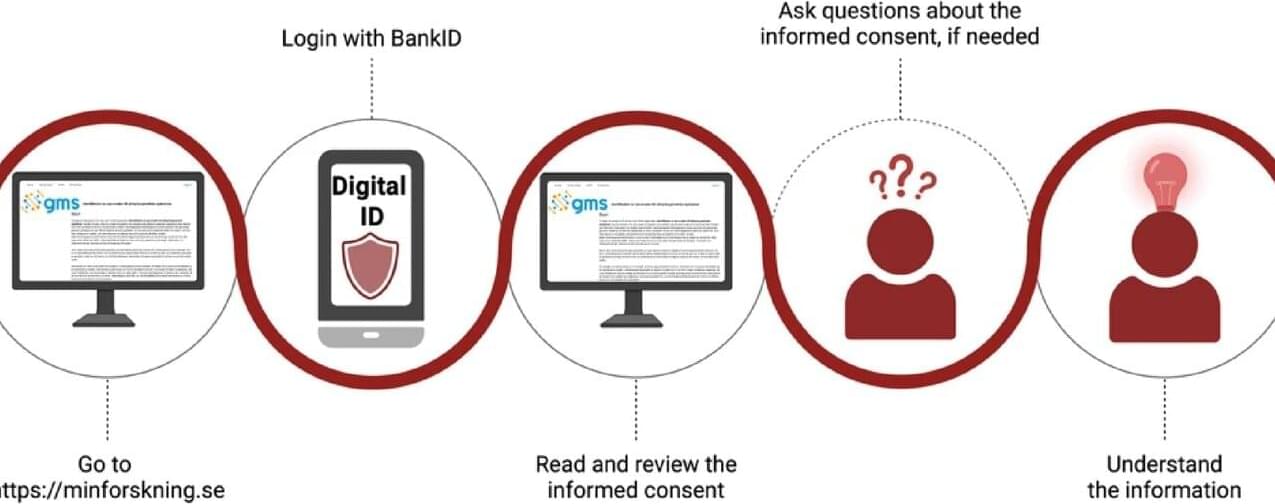
Research on rare diagnoses and the development of precision medicine depend on patients being able to share their health data in a secure and ethical manner. The research study, published in Scientific Reports, in which a digital platform was developed to collect electronic informed consent, shows that many participants want to contribute to research and appreciate the digital solution, but also that the technology needs further development.
A digital consent platform was tested at three centers in Sweden, Stockholm, Gothenburg, and Lund. More than 2,200 individuals who had previously undergone whole-genome sequencing were invited to give consent electronically for research and data sharing.
For those who lacked an electronic identity verification system, or who preferred traditional methods, paper-based consent was also available. As a comparison, a national patient cohort within Undiagnosed Diseases Network Sweden (UDN Sweden) was studied, where recruitment took place in close collaboration with patient organizations.