Transposable elements in cancer therapy.
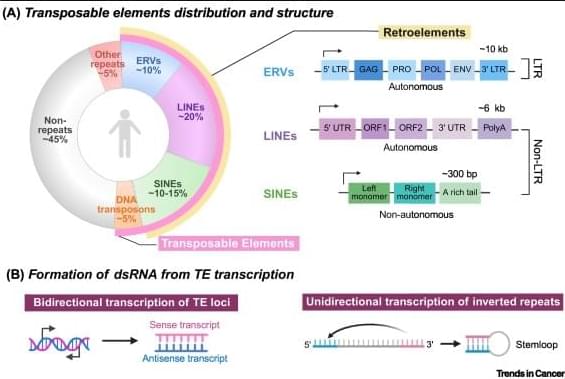
Transposable elements (TEs) are a major source of immunogenic nucleic acids that can be therapeutically reactivated in cancer cells to induce a state of viral mimicry.
TE expression can trigger innate immune sensing pathways, including type I interferon responses, and promote immunogenic cell death via sensors such as RIGI, MDA5, cGAS, and Z-DNA binding protein 1.
Although initially described in the context of epigenetic therapies, viral mimicry is now recognized as a shared response to diverse cancer treatment modalities, including chemotherapies and targeted therapies.
Despite their distinct primary mechanisms, these treatments converge on TE reactivation through disruption of DNA/histone methylation, p53 activation, and perturbation of mRNA splicing.
Therapeutic resistance to chemotherapy, radiation, and targeted agents is associated with TE silencing, identifying TE repression as a targetable axis of resistance.
Combination strategies to induce immunogenic TE expression can further enhance viral mimicry and boost antitumor immunity. https://sciencemission.com/Viral-mimicry-in-cancer-therapy
Viral mimicry is a cellular state in which the reactivation of silenced transposable elements (TEs) leads to the accumulation of immunogenic nucleic acids, triggering innate immune pathways that resemble responses mounted against viral pathogens. Although they were first characterized in the context of epigenetic therapies, growing evidence indicates that other cancer treatment modalities – including radiotherapy, chemotherapies, and targeted therapies – can also induce TE reactivation and viral mimicry responses in cancer cells. This review synthesizes the current knowledge on treatment-induced TE-mediated immune responses in cancer, highlighting therapeutic strategies, shared and distinct molecular mechanisms, and their broader implications for tumor–immune interactions and treatment outcomes.