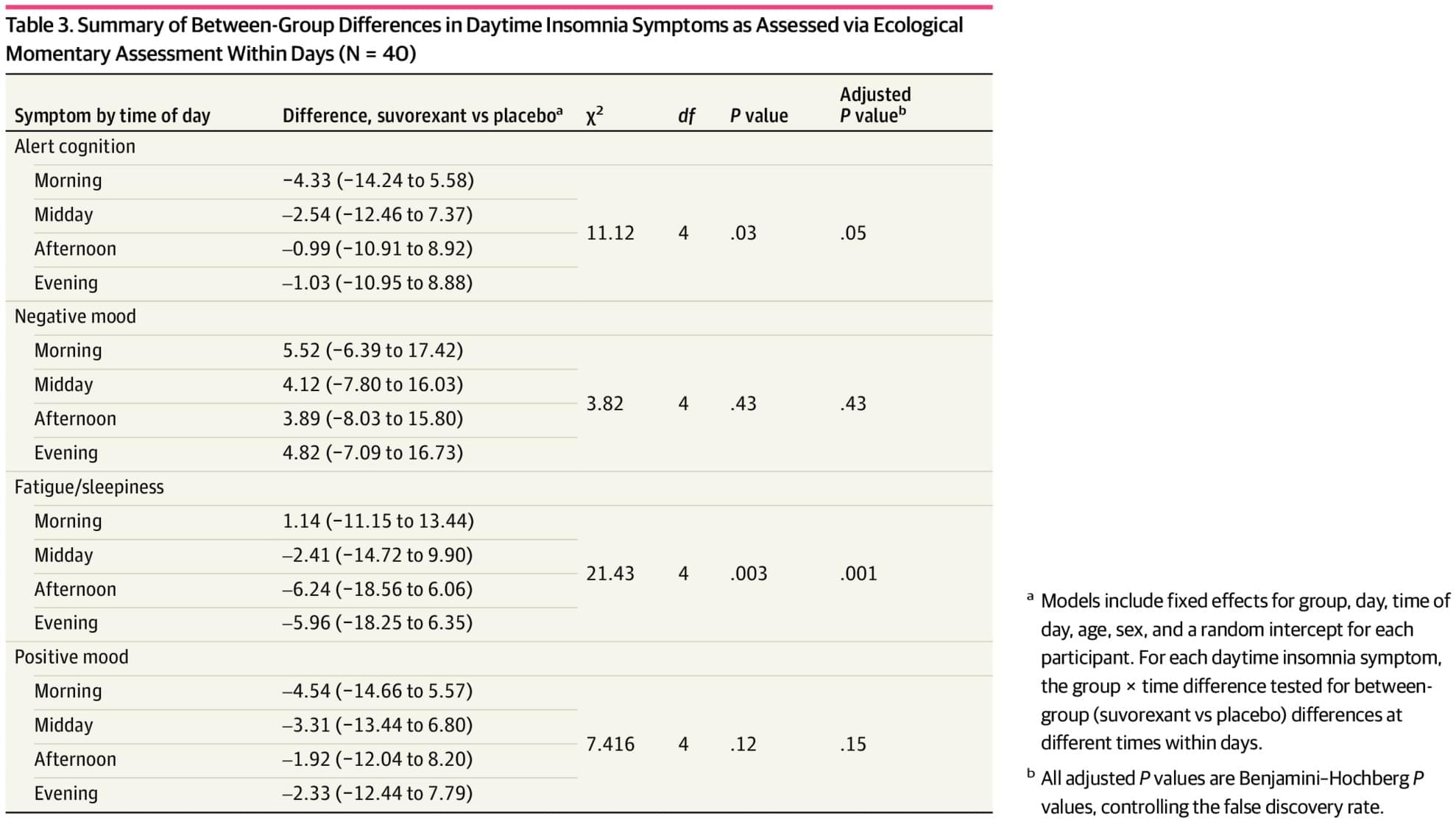
RCT: Smartphone-based ecological momentary assessment found greater morning fatigue but reduced afternoon and evening fatigue in patients with Insomnia treated with suvorexant vs placebo.
Question What is the effect of insomnia suvorexant pharmacotherapy on daytime insomnia symptoms as assessed via smartphone ecological momentary assessment (EMA)?
Findings In this randomized clinical trial that included 40 older adults with insomnia, traditional outcomes assessments detected differences between suvorexant and placebo groups in daytime insomnia symptoms; however, EMA was sensitive to detect effects of insomnia pharmacotherapy at various times of day.
Meaning These findings suggest that EMA warrants further refinement in sleep and psychiatric research and clinical care.