An interesting bioinformatic analysis which offers evidence suggesting that laboratory handling of AAVs may have contributed to horizontal gene transfer of the M-wide capsid across lineages in the wild. [ https://www.pnas.org/doi/10.1073/pnas.2505928122](https://www.pnas.org/doi/10.1073/pnas.2505928122)
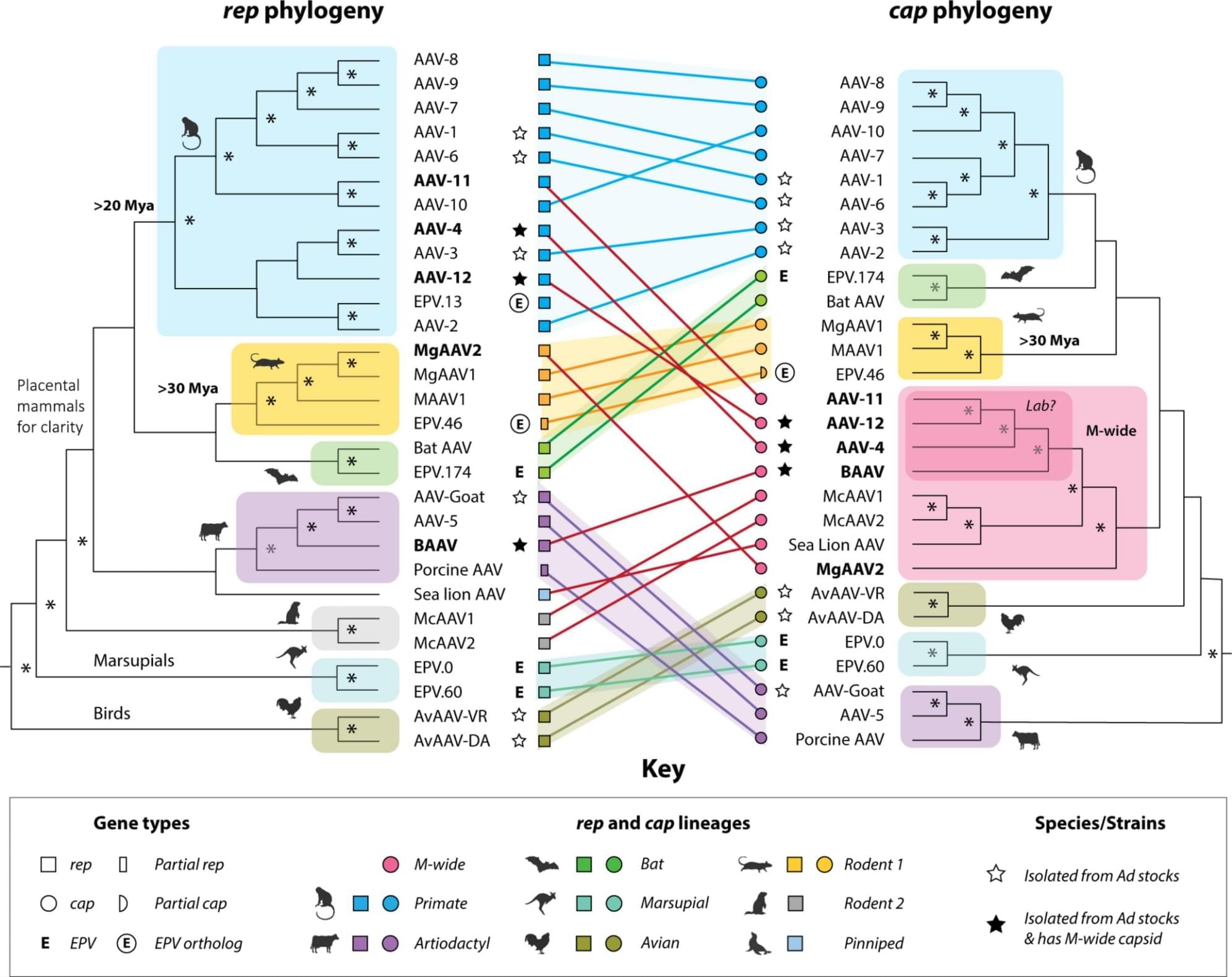
Adeno-associated viruses (AAVs) are nonpathogenic DNA viruses with potent gene delivery capabilities, making them essential tools in gene therapy and biomedical research. Despite their therapeutic importance, key aspects of AAV natural biology remain obscure, complicating efforts to explain rare AAV-associated diseases and optimize gene therapy vectors. By analyzing sequence data from virus isolates and endogenous viral elements (EVEs), I reveal a striking evolutionary pattern: While AAV sublineages, defined by the replication-associated (rep) gene, have broadly codiverged with host groups over millions of years, capsid (cap) diversity has been shaped by extensive recombination. In particular, one capsid lineage, Mammalian-wide (M-wide), has spread horizontally across diverse rep lineages and host taxa through multiple recombination events.