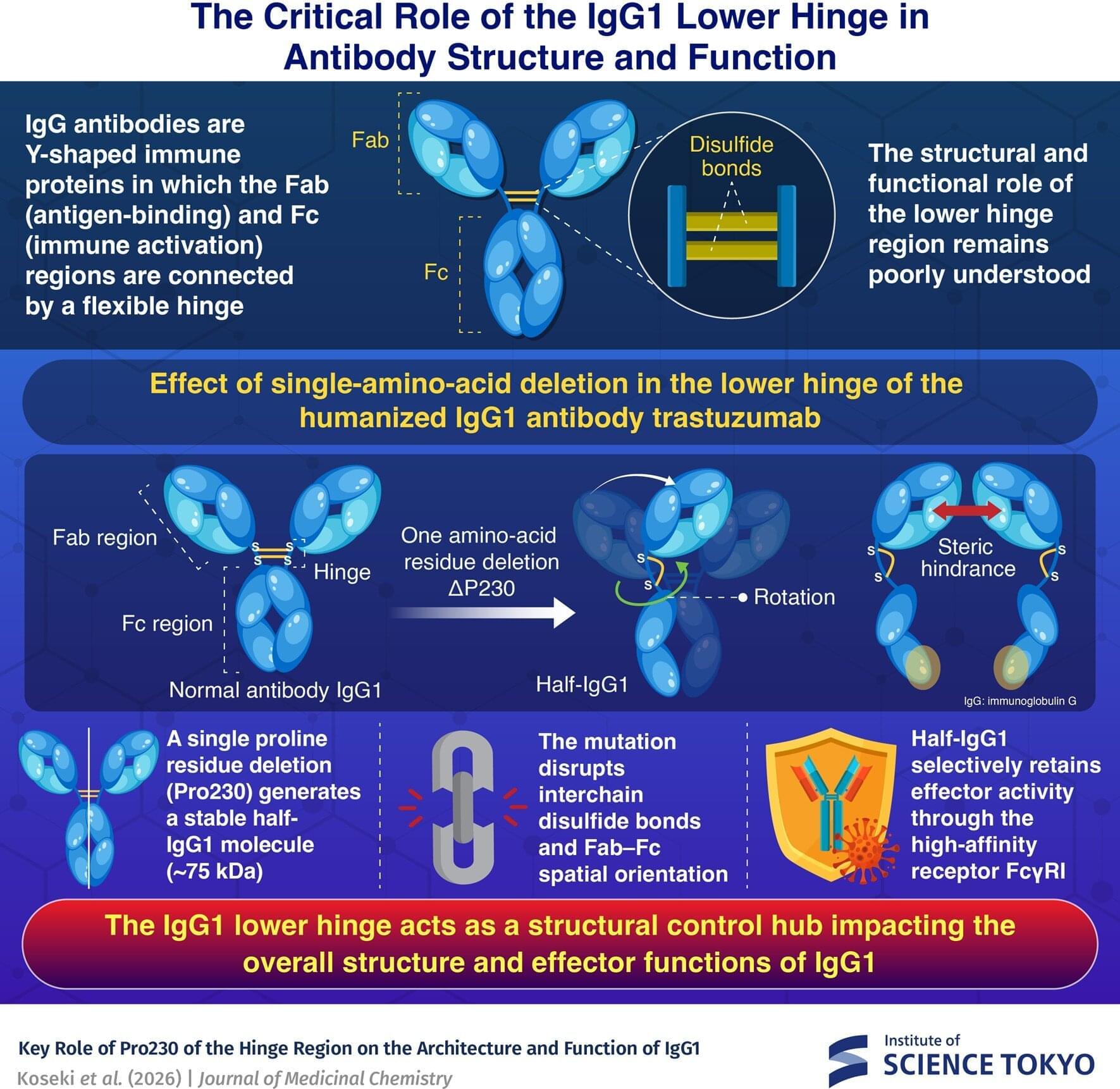
The lower hinge of immunoglobulin G (IgG), an overlooked part of the antibody, acts as a structural and functional control hub, according to a study by researchers at Science Tokyo. Deleting a single amino acid in this region transforms a full-length antibody into a stable half-IgG1 molecule with altered immune activity.
The findings provide a blueprint for engineering next-generation antibody therapies with precisely tailored immune effects for treating diseases such as cancer and autoimmune diseases.
Antibodies are Y-shaped proteins that help the immune system recognize and eliminate foreign threats such as bacteria and viruses. The dominant antibody in the bloodstream is immunoglobulin G (IgG), which accounts for about 75% of circulating antibodies. Its structure is divided into two main functional units connected by a flexible hinge that must work together seamlessly.