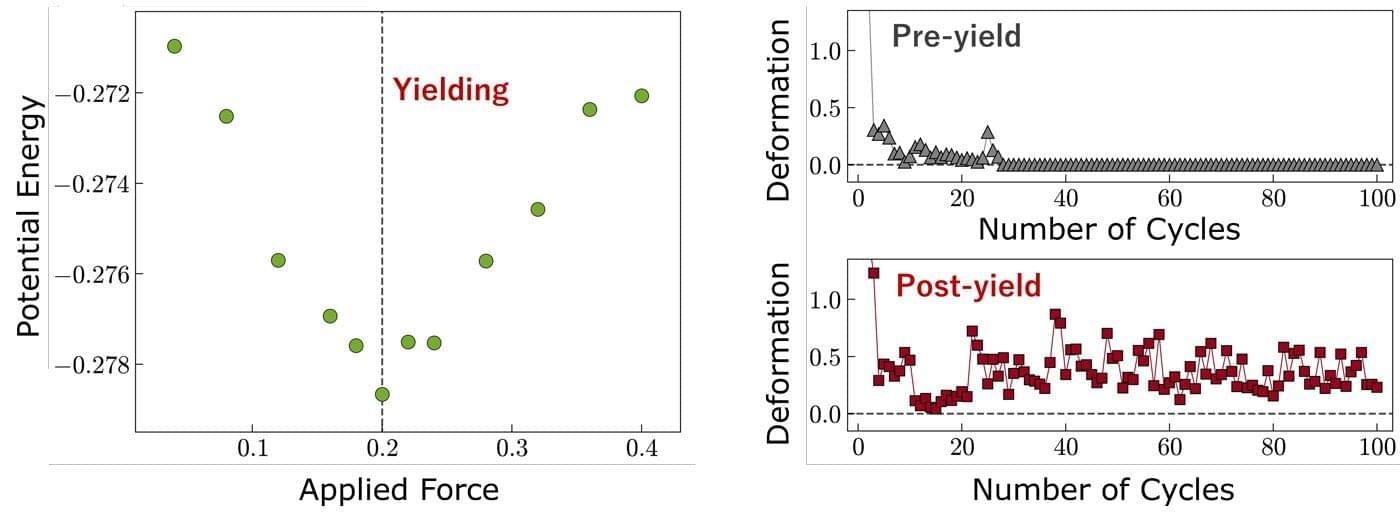
Glassy materials are everywhere, with applications far exceeding windowpanes and drinking glasses. They range from bioactive glasses for bone repair and amorphous pharmaceuticals that boost drug solubility to ultra-pure silica optics used in gravitational-wave detectors. In principle, any substance can become glass if its hot liquid is cooled fast enough to avoid forming an ordered crystal.
A distinguishing feature of glass is that its atoms freeze into an irregular, disordered arrangement. This stands in contrast to crystals, where atoms sit in a regular pattern. This disorder gives glass many of its unique and useful properties, but scientists still struggle to understand how atomic-scale disorder produces the properties observed in everyday glasses.