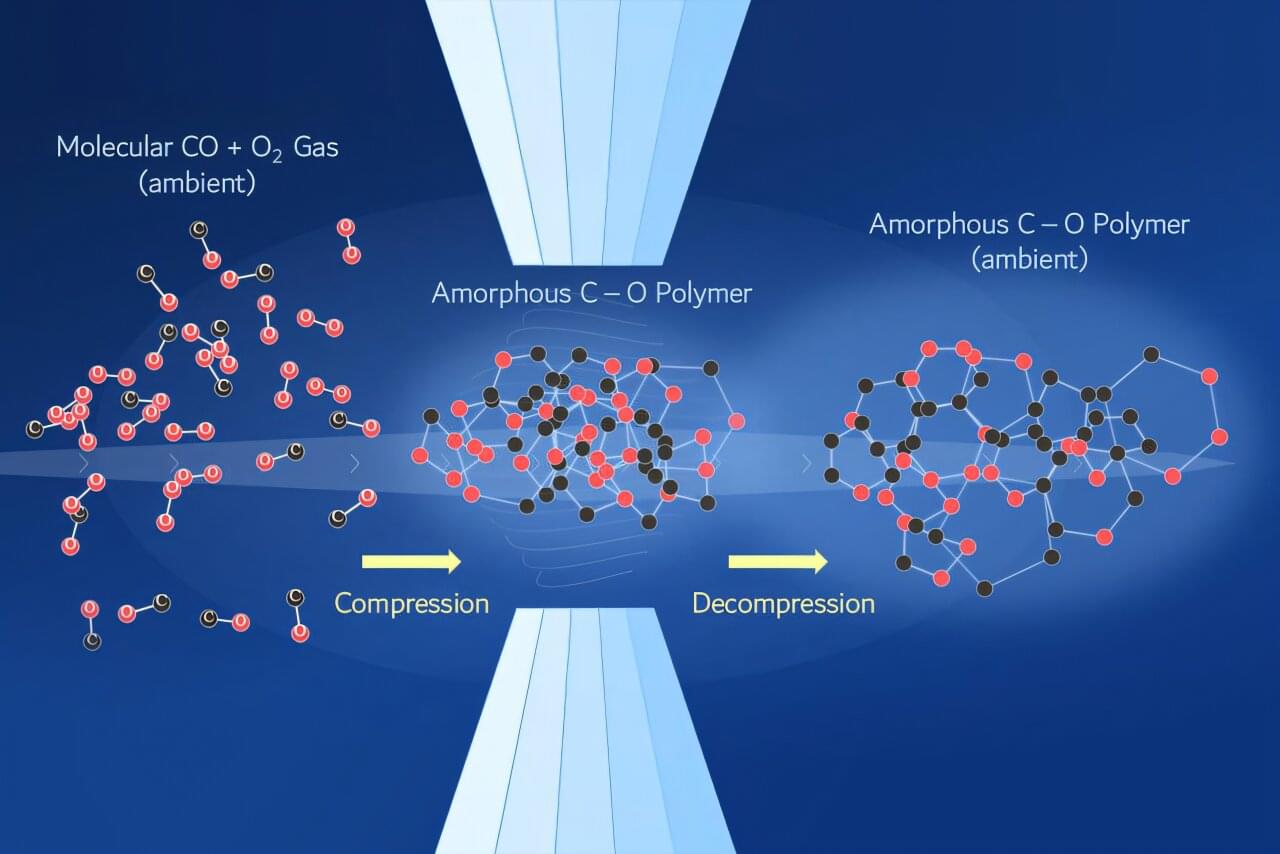
When materials are compressed, their atoms are forced into unusual arrangements that do not normally exist under everyday conditions. These configurations are often fleeting: when the pressure is released, the atoms typically relax back to a stable low-pressure state. Only a few very specific materials, like diamond, retain their high-pressure structure after returning to room temperature and atmospheric pressure.
But locking those atomic arrangements in place under ambient conditions could create new classes of useful materials with a wide range of potential applications. One particularly compelling example is energetic materials, which are useful for propellants and explosives.
In a study published in Communications Chemistry, researchers at Lawrence Livermore National Laboratory (LLNL) identified a first-of-its-kind carbon dioxide-equivalent polymer that can be recovered from high-pressure conditions.