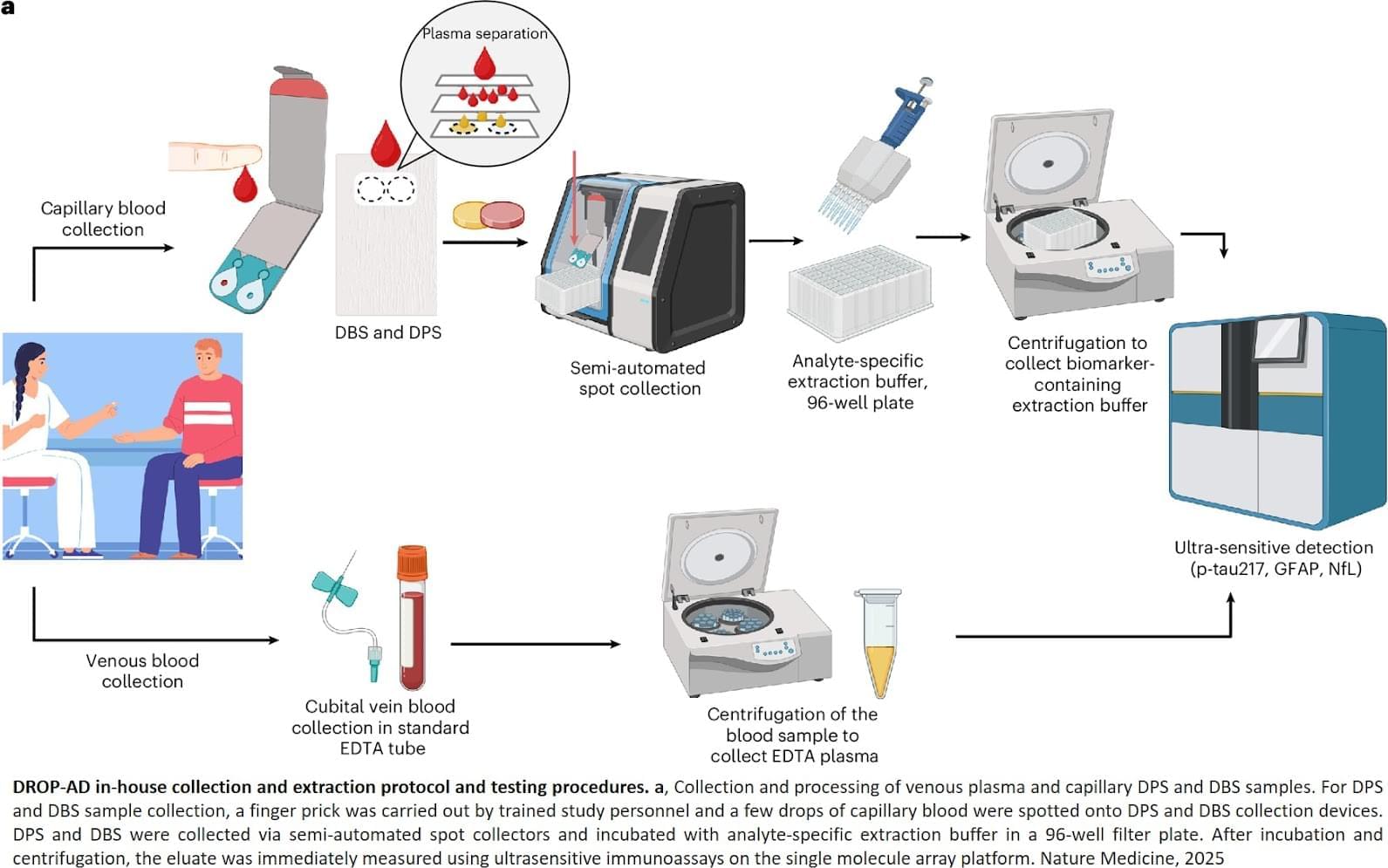
The researchers tested a new method for detecting Alzheimer’s disease using a few drops of blood obtained from the fingertip and then dried on a card. This process was used to find proteins linked to Alzheimer’s disease and other brain changes in the 337 participants.
The study found that levels of p-tau217 in finger-prick samples closely matched results from standard blood tests and were able to identify Alzheimer’s disease-related changes in spinal fluid with an accuracy of 86 per cent. Two other markers, glial fibrillary acidic protein (GFAP) and neurofilament light (NfL), were also successfully measured and showed strong agreement with traditional tests.
While not ready for clinical use, this breakthrough addresses critical barriers in Alzheimer’s research by enabling remote participation in studies, clinical trial recruitment and monitoring, broader population sampling for epidemiological research, and inclusion of underrepresented communities and regions with limited healthcare infrastructure.
The findings suggest that this simple technique could make large-scale studies and remote testing possible, including for people with Down syndrome, who face a higher risk of Alzheimer’s disease and for other underserved populations. ScienceMission sciencenewshighlights.
A groundbreaking international study has demonstrated that Alzheimer’s disease biomarkers can be accurately detected using simple finger-prick blood samples that can be collected at home and mailed to laboratories without refrigeration or prior processing.
The research published in Nature Medicine. It represents the first large-scale validation of this accessible testing approach that removes geographic barriers and opens brain disease research to global populations without requiring specialised healthcare infrastructure.
The researchers successfully tested 337 participants and proved that finger-prick blood collection can accurately measure key markers of Alzheimer’s pathology and brain damage. This breakthrough enables worldwide research participation by eliminating the logistical constraints that have historically limited biomarker studies to well-resourced medical facilities.