Metabolic control of antitumor Immunity in neuroblastoma👇
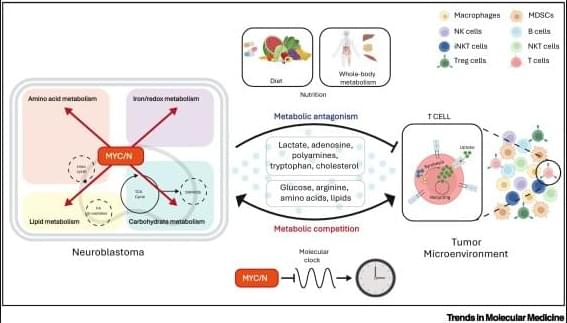
✅Distinct metabolic TMEs in MNA vs non-MNA tumors Neuroblastoma (NB) tumors display markedly different tumor microenvironments depending on MYCN amplification (MNA) status. MYCN acts as a key driver of metabolic remodeling, reshaping how nutrients and metabolites are distributed within the TME.
✅MYCN-amplified NB: immunosuppressive metabolism In MNA neuroblastoma, tumor cells aggressively consume shared metabolites such as glucose, glutamine, methionine, cysteine, and lipids. This metabolic competition deprives infiltrating T cells of essential nutrients, promoting T cell exhaustion and dysfunction. In parallel, MNA tumor cells release antagonistic metabolites, including lactate, adenosine, and cholesterol, which further suppress TCR signaling, proliferation, and effector functions.
✅T cell exhaustion and impaired tumor killing Within the MNA TME, T cells exhibit exhausted phenotypes characterized by impaired proliferation, altered STAT5 signaling, and reduced cytotoxic activity. This metabolic and signaling imbalance leads to ineffective immune-mediated tumor killing.
✅Non-MNA NB: permissive immune landscape In contrast, non-MNA neuroblastomas do not impose the same metabolic constraints. Nutrient availability is better preserved, allowing cytotoxic T cells to infiltrate tumors, maintain effector functions, and induce effective tumor cell death.
✅Biological and therapeutic implications These findings highlight metabolism as a central regulator of antitumor immunity in neuroblastoma. Targeting MYCN-driven metabolic pathways may help restore T cell function and improve the efficacy of immunotherapies, particularly in MYCN-amplified disease.
Oncogenic MYCN drives aggressive disease in many cancers including neuroblastoma (NB). Metabolic reprogramming is essential to support cancer cell homeostasis and survival under nutrient-and oxygen-deprived conditions. MYCN directly reprograms many nodes of tumor-intrinsic metabolism, which have significant repercussions on the cells of the tumor microenvironment (TME), resulting in complex intercellular metabolic circuits that contribute to the immunosuppressive microenvironment of NB. These metabolic circuits are also regulated by the organismal and cellular circadian clock and host diet to further impact the TME and NB oncogenesis. This review discusses the mechanisms by which MYCN regulates the metabolic crosstalk between tumor, TME, and host, and provides evidence that therapeutic targeting of MYCN-reprogrammed metabolism can improve patient outcomes.