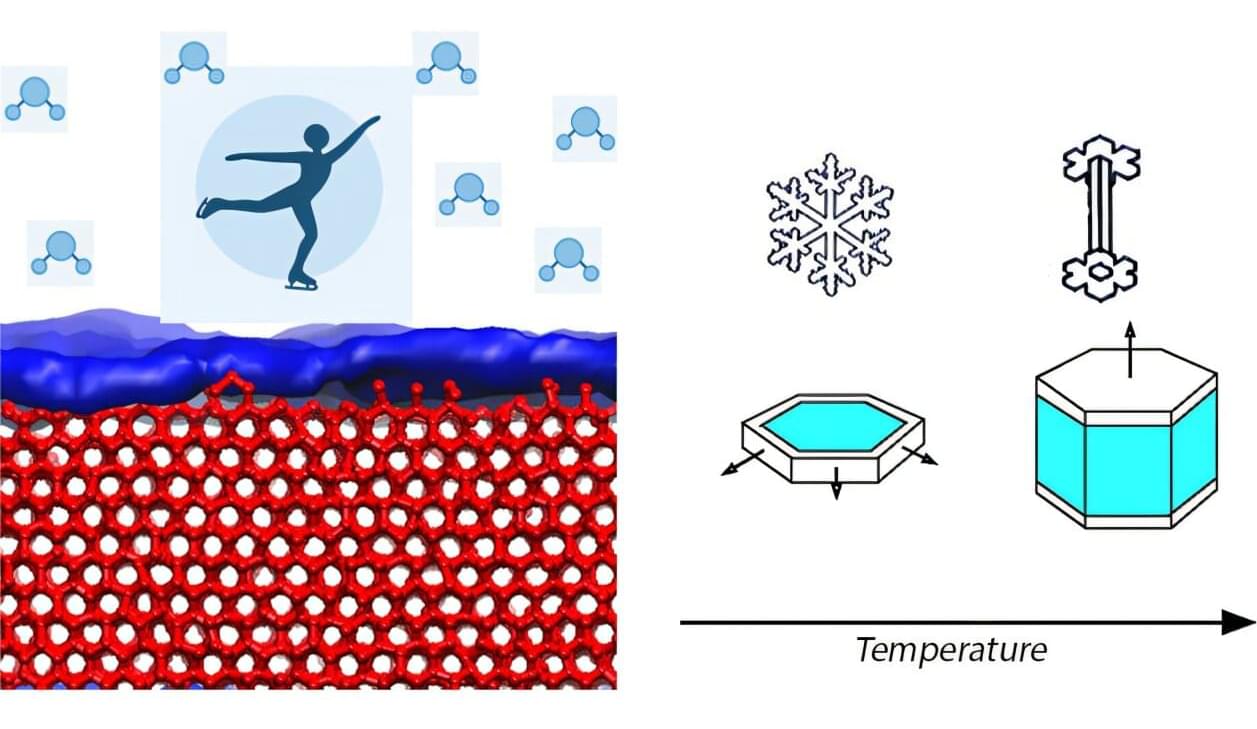
The ice in a domestic freezer is remarkably different from the single crystals that form in snow clouds, or even those formed on a frozen pond. As temperatures drop, ice crystals can grow in a variety of shapes: from stocky hexagonal prisms to flat plates, to Grecian columns.
Why this structural roller coaster happens, though, is a mystery. When first observed, researchers thought it must relate to a hypothesis proposed by famed physicist Michael Faraday—ice below its melting point has a microscopically thin liquid layer of water across its surface.
This “premelting film” of ice, however, is the subject of significant scientific controversy. For years, researchers have provided contradictory evidence about its thickness and whether it even exists.