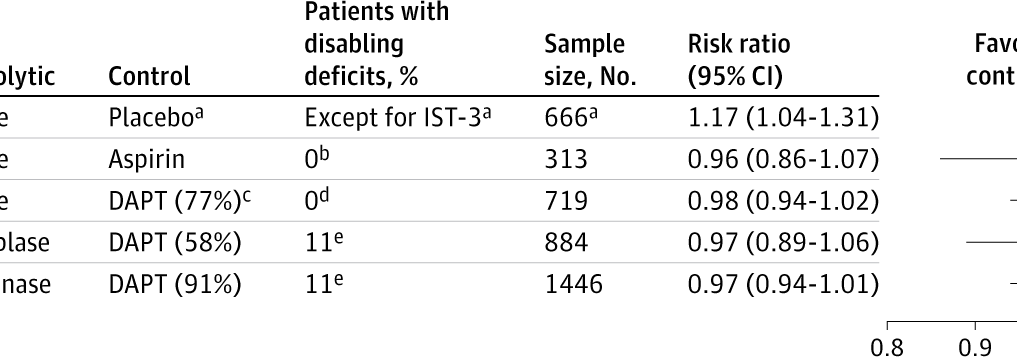
A secondary analysis of the TEMPO-2 RCT found no significant improvement in outcomes for minor ischemic stroke patients treated with intravenous tenecteplase, regardless of the presence of disabling deficits.
Question Did outcomes following intravenous tenecteplase for minor ischemic stroke vary based on the presence of disabling deficits?
Findings In this secondary analysis of the TEMPO-2 randomized clinical trial including 884 patients with minor ischemic stroke and proven intracranial occlusion, both patients with and without disabling deficits defined according to US National Institutes of Health Stroke Scale (NIHSS)–based criteria showed a neutral treatment effect from intravenous tenecteplase, with no significant effect modification.
Meaning Current definitions of disabling stroke did not modify the neutral treatment effect of intravenous tenecteplase in patients with minor stroke and intracranial occlusion.