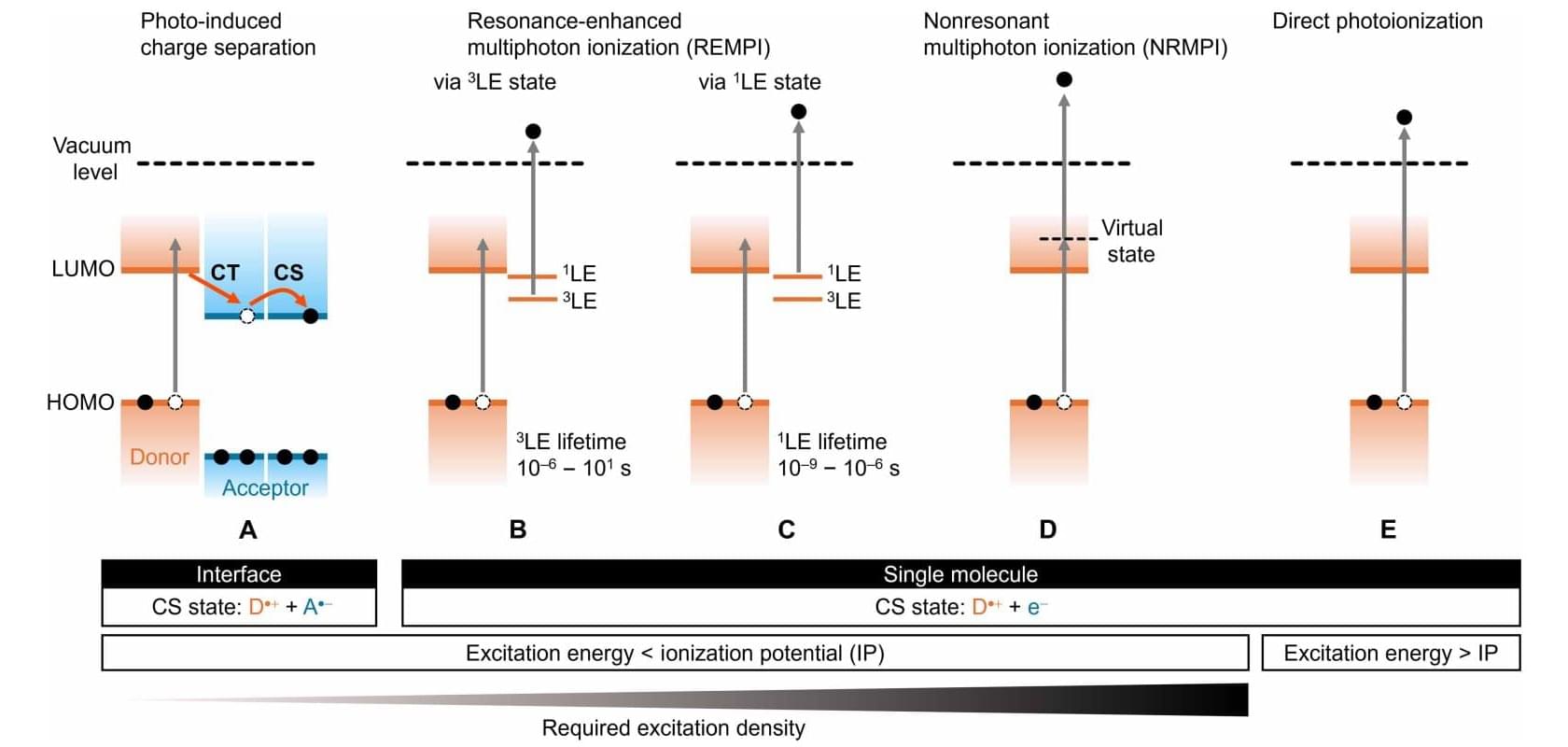
Why does plastic turn brittle and paint fade when exposed to the sun for long periods? Scientists have long known that such organic photodegradation occurs due to the sun’s energy generating free radicals: molecules that have lost an electron to sunlight-induced ionization and have been left with an unpaired one, making them very eager to react with other molecules in the environment. However, the exact mechanisms for how and why the energy from the sun’s photons get stored and released in the materials over very long periods have eluded empirical evidence.
The problem lies in the timeframe. While scientists have access to extremely sophisticated spectroscopy equipment capable of measuring the energy levels of individual electrons at femtosecond to millisecond scales in organic materials, they have paid little attention to time scales beyond seconds—and these are processes that can take years.
As such, slow, transient charge accumulation has presented a disappointing data gap in both applied and theoretical optics. But now, researchers from the Organic Optoelectronics Unit at the Okinawa Institute of Science and Technology (OIST) have addressed this challenge with a new methodology that detects these faint signals. Their findings are published in Science Advances.