In a 4-year analysis of the NATALEE trial, adjuvant ribociclib plus NSAI continued to provide invasive disease-free survival benefit over NSAI alone in patients with hormone receptor–positive/ERBB2-negative early breast cancer, with stable safety outcomes observed.
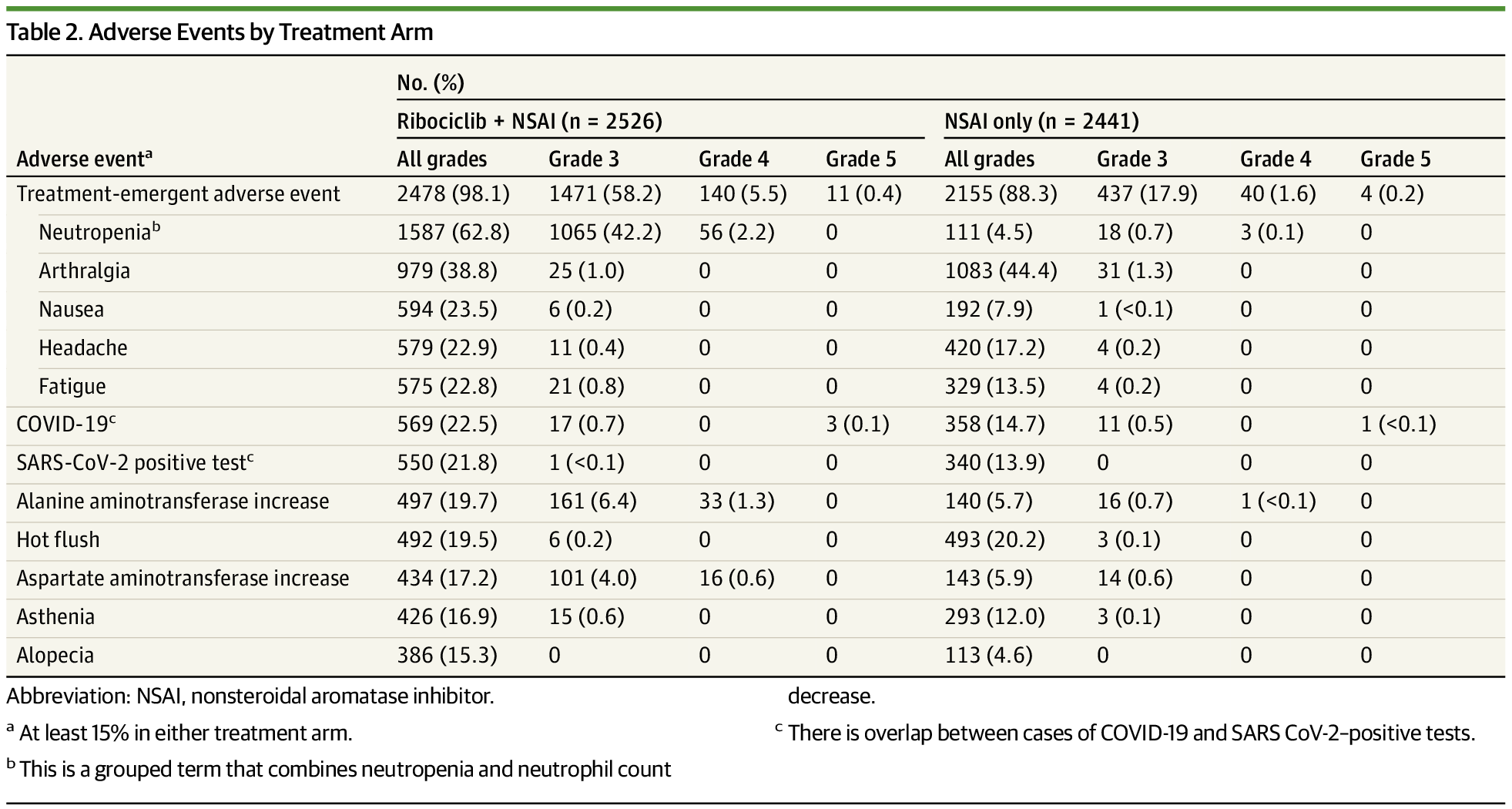
This exploratory 4-year analysis demonstrated a consistent iDFS benefit with ribociclib plus NSAI with additional follow-up, with a 28.5% reduction in the risk of recurrence over NSAI alone and confidence intervals that continue to narrow. The absolute improvement in iDFS with ribociclib plus NSAI continued to increase from 2.7% at 3 years to 4.9% at 4 years. The iDFS, DDFS, RFS, and DRFS benefits were observed with ribociclib plus NSAI over NSAI alone beyond the planned 3-year ribociclib treatment duration, as all patients were no longer receiving ribociclib treatment at the data cutoff. A sustained iDFS benefit with ribociclib plus NSAI was seen across subgroups, including anatomic stage and nodal status, with a consistent reduction in risk of distant recurrences across subgroups. The safety profile remained unchanged with additional follow-up.
In prior analyses, an iDFS benefit was observed with ribociclib plus NSAI over NSAI alone.10,21 However, at the time of those analyses, all patients had not yet discontinued ribociclib treatment (54.0% in the second interim efficacy analysis and 78.3% in the protocol-specified final analysis were no longer receiving treatment).10,21 Thus, the efficacy of ribociclib beyond the planned treatment period was unknown. In this analysis, all patients had stopped ribociclib treatment, with 62.8% of patients having completed the planned 3-year treatment duration. At 4 years, the absolute improvement in iDFS increased and the Kaplan-Meier curves continue to separate with additional follow-up, suggesting a persistent benefit of adjuvant ribociclib beyond the planned 3-year treatment duration.
An iDFS benefit was observed with ribociclib plus NSAI over NSAI alone in patients with high-risk N0 disease. While this subgroup of patients in NATALEE do not have nodal involvement, enrolled patients with stage IIA node-negative (T2N0) disease were required to have additional high-risk features, including grade 3 disease or grade 2 disease with Ki-67 20% or higher or high genomic risk. Previous meta-analyses and population-based studies have shown risk of recurrence across patients with and without nodal involvement; however, many of the patient samples in these studies were enrolled decades prior to the follow-up analyses.5, 24 The cohorts in these long-term studies may not be reflective of contemporary treatment standards, which has the potential to overinflate the recurrence risk among some subgroups.