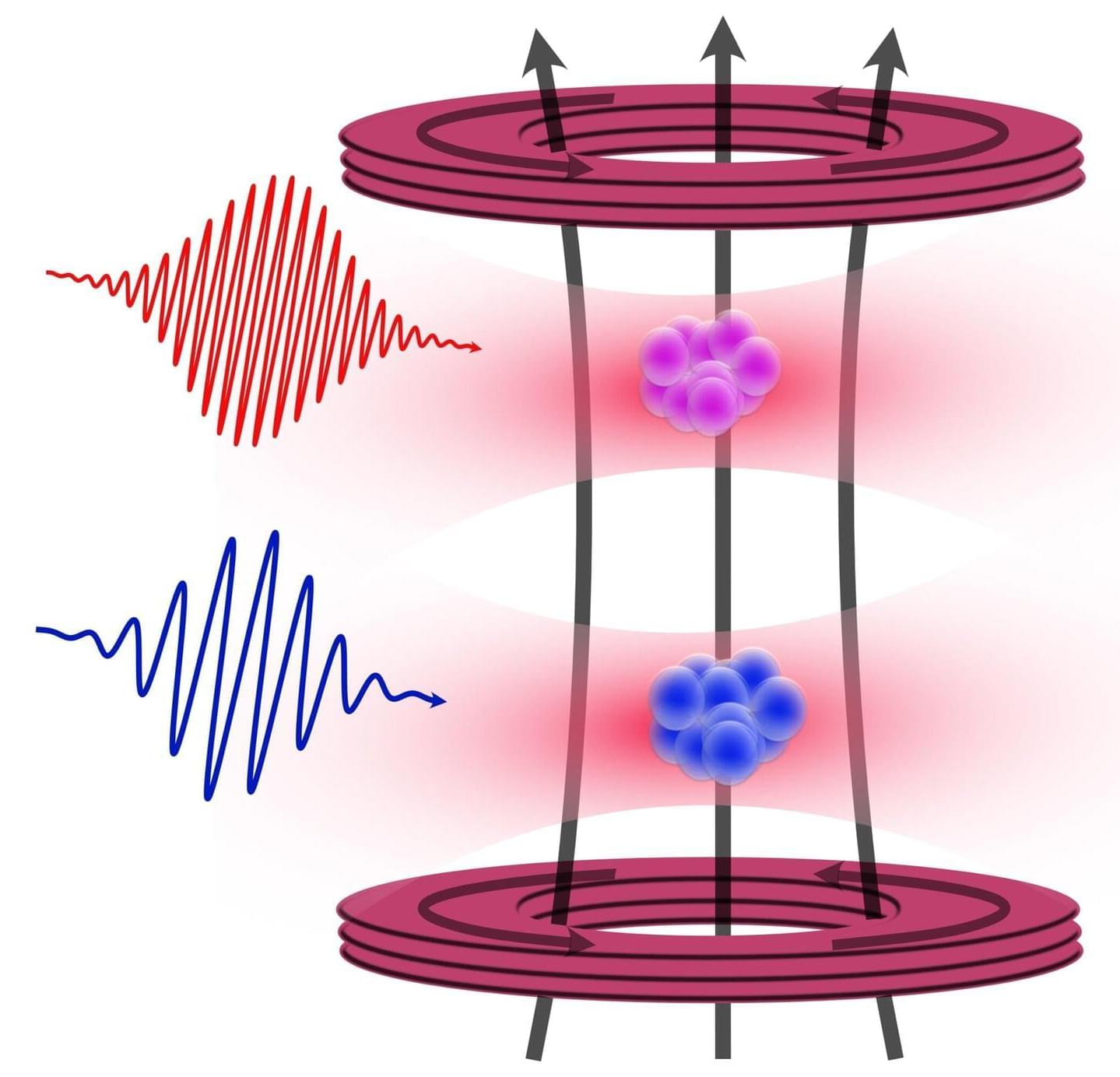
Having good neighbors can be very valuable—even in the atomic world. A team of Amsterdam physicists was able to determine an important property of strontium atoms, a highly useful element for modern applications in atomic clocks and quantum computers, to unprecedented precision. To achieve this, they made clever use of a nearby cloud of rubidium atoms. The results were published in the journal Physical Review Letters this week.
Strontium. It is perhaps not the most popularly known chemical element, but among a group of physicists it has a much better reputation—and rightfully so.
Strontium is one of six so-called alkaline earth metals, meaning that it shares properties with better-known cousins like magnesium, calcium and radium. Strontium atoms have 38 protons in their nucleus, and a varying number of neutrons—for the variations (or isotopes) of strontium that can be found in nature, either 46, 48, 49 or 50.