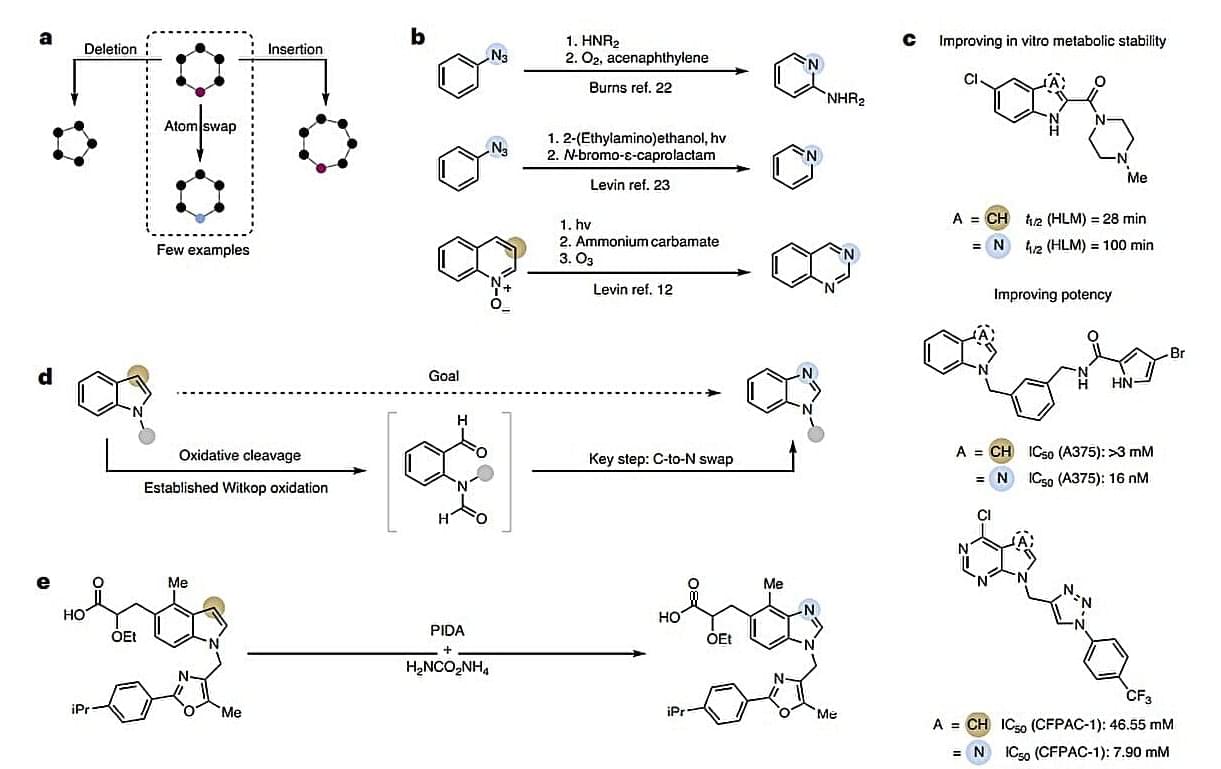
Scientists have achieved a new feat in molecular editing by swapping carbon for nitrogen, enabling the direct conversion of indoles into benzimidazoles. This simple switch in a one-pot method offers a hassle-free and effective way of designing medicinally relevant molecules. The work is published in Nature Chemistry.
Single-atom swap reactions require the selective formation and breaking of multiple bonds at the same time, making them quite rare and challenging.
Researchers from ETH Zurich overcame these hurdles by exploiting the electron-rich indole ring’s eagerness to undergo oxidative cleavage via Witkop oxidation. This step can split the electron-rich ring open to form a dicarbonyl intermediate, thereby creating an entry point for subsequent cascade reactions.