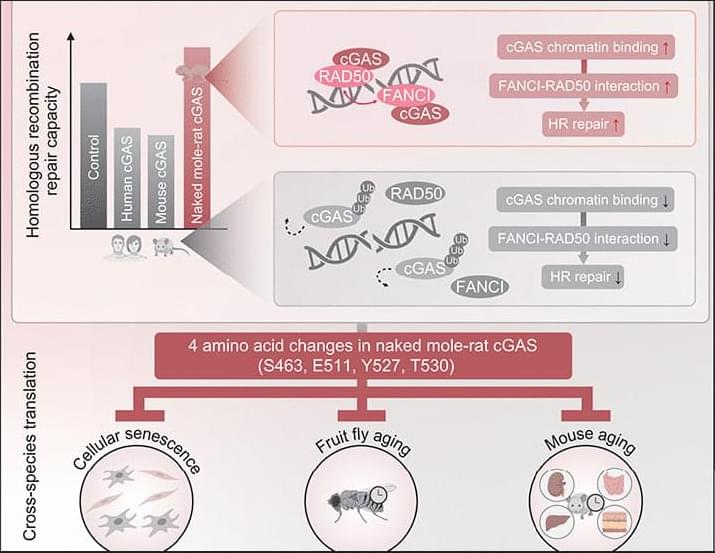
Efficient DNA repair might make possible the longevity of naked mole-rats. However, whether they have distinctive mechanisms to optimize functions of DNA repair suppressors is unclear. We find that naked mole-rat cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS) lacks the suppressive function of human or mouse homologs in homologous recombination repair through the alteration of four amino acids during evolution. The changes enable cGAS to retain chromatin longer upon DNA damage by weakening TRIM41-mediated ubiquitination and interaction with the segregase P97. Prolonged chromatin binding of cGAS enhanced the interaction between repair factors FANCI and RAD50 to facilitate RAD50 recruitment to damage sites, thereby potentiating homologous recombination repair.