All-solid-state batteries are safe, powerful ways to power EVs and electronics and store electricity from the energy grid, but the lithium used to build them is rare, expensive and can be environmentally devastating to extract.
Sodium is an inexpensive, plentiful, less-destructive alternative, but the all-solid-state batteries they create currently don’t work as well at room temperature.
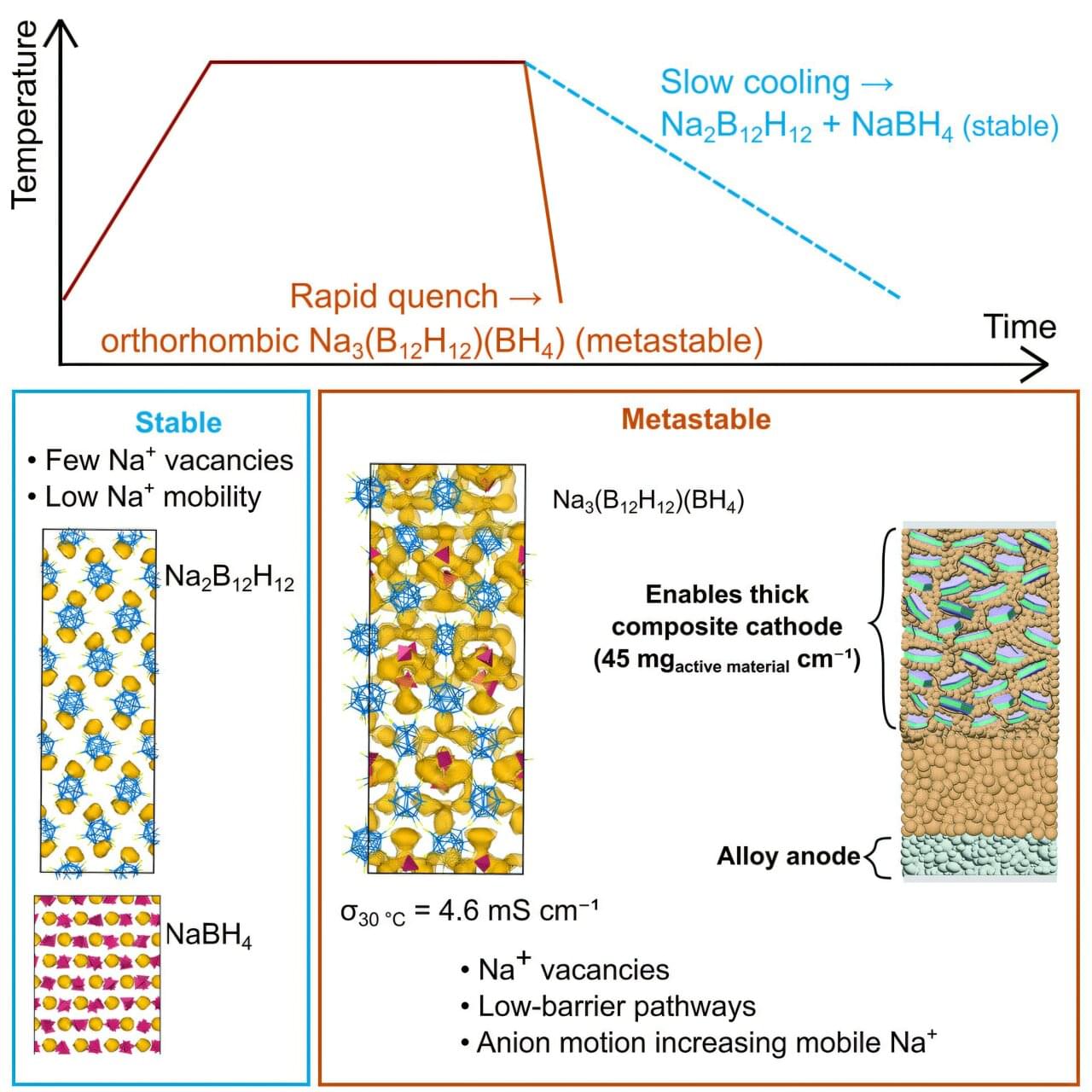
“It’s not a matter of sodium versus lithium. We need both. When we think about tomorrow’s energy storage solutions, we should imagine the same gigafactory can produce products based on both lithium and sodium chemistries,” said Y. Shirley Meng, Liew Family Professor in Molecular Engineering at the UChicago Pritzker School of Molecular Engineering (UChicago PME). “This new research gets us closer to that ultimate goal while advancing basic science along the way.”