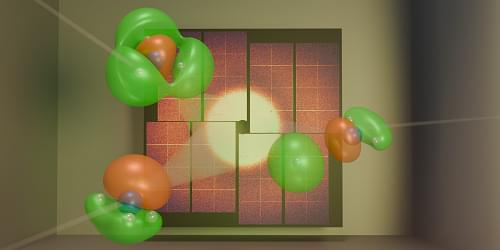
Scientists have used ultrashort x-ray pulses to directly observe the motion of electrons driving a chemical reaction.
A chemical reaction occurs when chemical bonds break and new ones form. These bonds hold atoms together within molecules and are governed by the atoms’ outermost electrons. The motion of these so-called valence electrons dictates how a reaction starts and determines its final products. For decades, chemists have envisioned the possibility of watching such electron movement in real time, capturing a movie of valence electrons as bonds break and form. Now Ian Gabalski at Stanford University and his colleagues have brought this dream closer to reality [1]. They have observed valence-electron motion occurring within a few hundred femtoseconds—where one femtosecond is a millionth of a billionth of a second. This feat was accomplished using ultrashort, high-energy x-ray pulses produced at SLAC National Accelerator Laboratory in California. The team’s findings provide an intuitive view of how electron dynamics influence chemical reactions.
Directly observing electron motion during chemical reactions presents two main challenges. First, it requires an imaging technique that can map the spatial distribution of electrons, known as the electron density. This distribution spans only a few tenths of a nanometer, demanding extremely high spatial resolution. Second, the task needs ultrahigh temporal resolution, because electron movement occurs on a timescale of femtoseconds or even attoseconds—thousandths of a femtosecond. Capturing such rapid motion requires the sample to be subjected to light pulses that are short enough to effectively freeze electron dynamics in time, similarly to using a high-speed camera to capture the fluttering wings of a hummingbird.