Adjuvant immunotherapy plus chemotherapy has yet to square off against neoadjuvant immunotherapy in a head-to-head trial. Even if that trial doesn’t happen, post hoc analyses of ATOMIC and the neoadjuvant NICHE-2 studies may clarify whether a one-size-fits-all approach is appropriate and help determine which patients benefit more from one approach over the other, according to Christopher Lieu, MD, an investigator in the ATOMIC study.
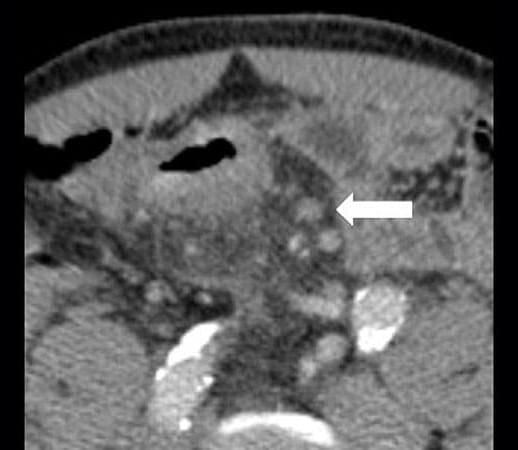
The ATOMIC study showed that adding adjuvant immunotherapy to standard-of-care chemotherapy following resection reduced the risk for disease recurrence or death by 50% compared with chemotherapy alone in the 355 patients with stage III colon cancer with mismatch repair deficiency (dMMR), who received adjuvant atezolizumab along with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy, providing those in the pro-adjuvant camp with important data. In addition, 3-year disease-free survival (DFS) was 86.4% with the combination compared with 76.6% with chemotherapy alone. The results of this trial were presented at the American Society of Clinical Oncology (ASCO) 2025.
Experts debate which patients with stage III dMMR colon cancer will benefit from the two treatment approaches.