Compared with the energy-intensive Haber-Bosch process, renewable energy-driven electrocatalytic nitrate reduction reaction (NO3−RR) provides a low-carbon route for ammonia synthesis under mild conditions. Using nitrate from wastewater as the nitrogen source and water as the hydrogen source, this route has the potential to produce ammonia sustainably while mitigating water pollution.
Copper (Cu)-based catalysts show a good performance for NO3−RR to ammonia. However, they suffer from issues including high overpotential, competing nitrite (NO2–) formation, and low overall energy efficiency.
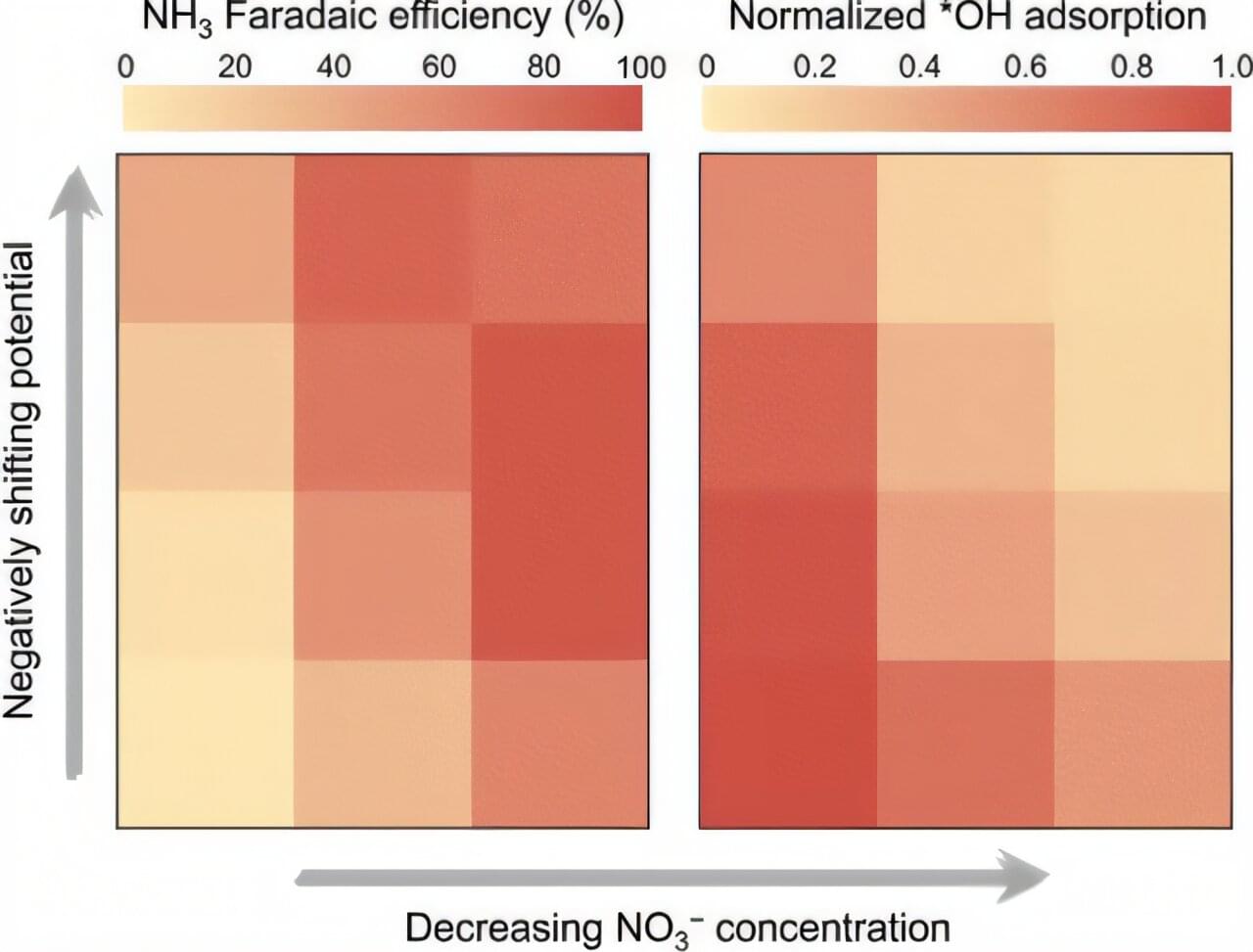
In a study published in ACS Catalysis, a team led by Prof. Bao Xinhe and Prof. Gao Dunfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, along with Prof. Wang Guoxiong from Fudan University, proposed hydroxyl (*OH) adsorption as a selectivity descriptor for ammonia synthesis via NO3−RR over Cu catalysts.