The graphite found in your favorite pencil could have instead been the diamond your mother always wears. What made the difference? Researchers are finding out.
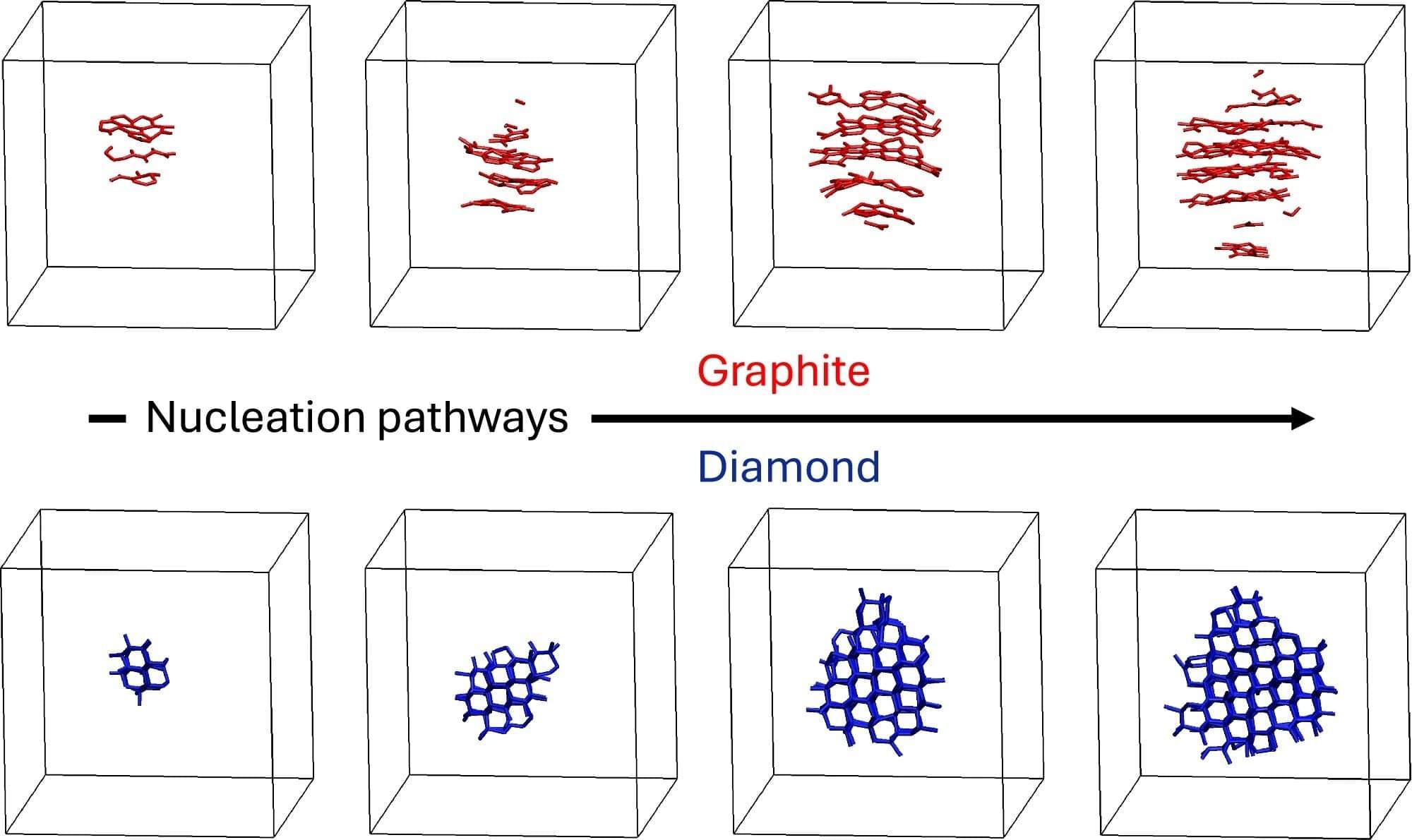
How molten carbon crystallizes into either graphite or diamond is relevant to planetary science, materials manufacturing and nuclear fusion research. However, this moment of crystallization is difficult to study experimentally because it happens very rapidly and under extreme conditions.
In a new study published July 9 in Nature Communications, researchers from the University of California, Davis and George Washington University use computer simulations to study how molten carbon crystallizes into either graphite or diamond at temperatures and pressures similar to Earth’s interior. The team’s findings challenge conventional understanding of diamond formation and reveal why experimental results studying carbon’s phase behavior have been so inconsistent.