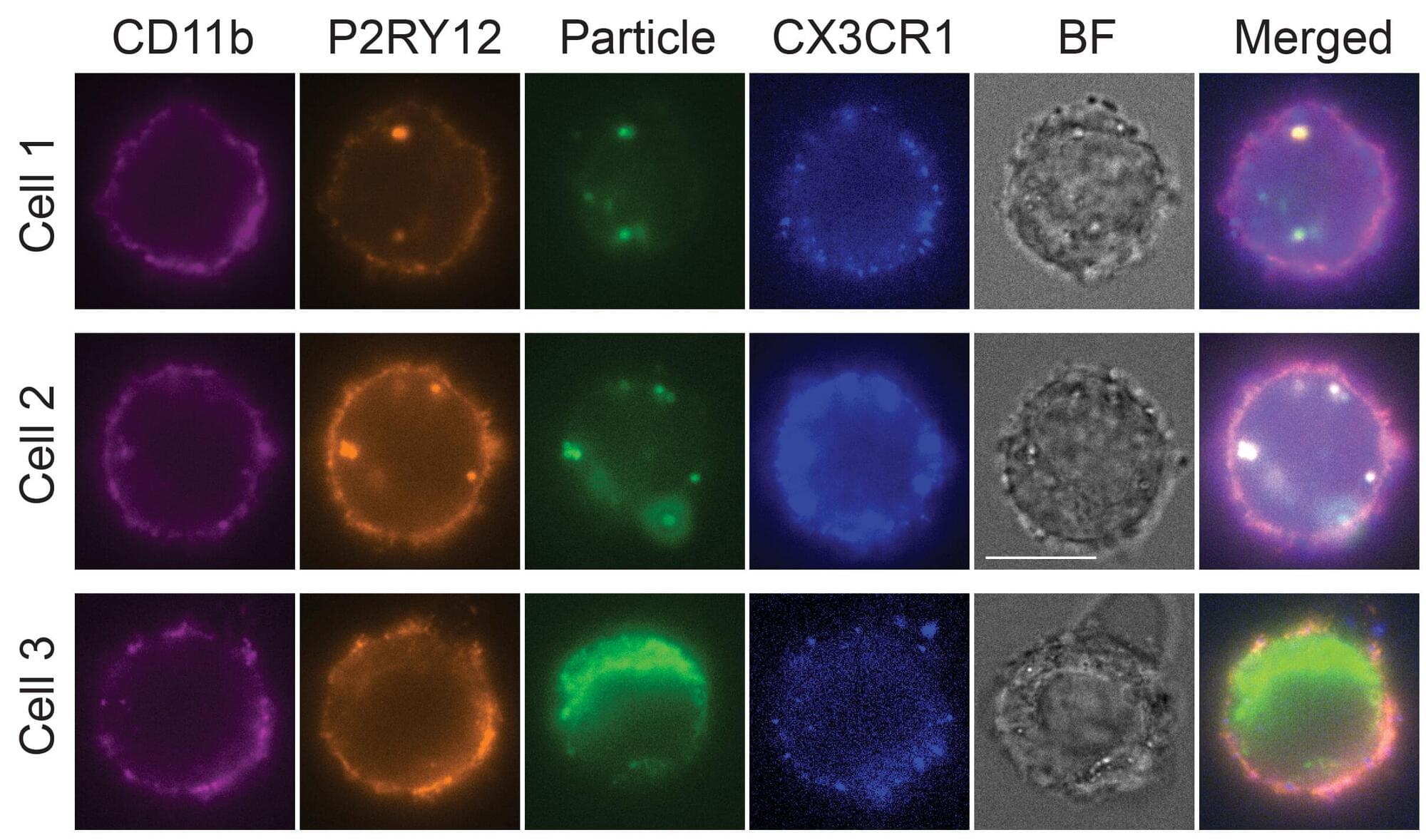
Microglia are a specialized type of immune cell that accounts for about 10% of all cells within the brain and spinal cord. They function by eliminating infectious microbes, dead cells, and aggregated proteins, as well as soluble antigens that may endanger the brain and, during development, also help shape neural circuits enabling specific brain functions.
When microglia don’t function properly, they can trigger neuroinflammation and fail to clear away damaged cells and harmful protein clumps—such as the neurofibrillary tangles and amyloid plaques seen in Alzheimer’s disease. This contributes to numerous neurodegenerative diseases, including Alzheimer’s, Parkinson’s and Huntington’s disease, as well as amyotrophic lateral sclerosis (ALS), multiple sclerosis, and other disorders. In fact, neuroinflammation can occur even before proteins start to form pathogenic aggregates and, in turn, accelerates protein aggregation.
Researchers and drug developers aiming to better understand and target microglia functions in the brain are challenged by the fact that human microglia can only be obtained through biopsies, and rodents’ microglia differ from their human counterparts in many critical features. This supply issue prompted them to work on methods to create microglia in the culture dish using stem cells as a starting point. However, to date, this process has remained inefficient, and requires weeks to complete at significant costs.